Free pharmacy material


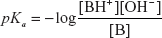
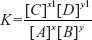
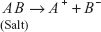
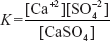
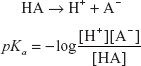
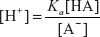
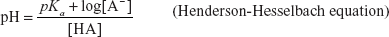
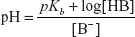
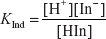
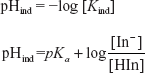

Acid-base Titrations
(Neutralization Titrations)
INTRODUCTION
In 1887 Arrhenius proved that the acids produce the hydrogen ions on ionization. These hydrogen ions are first electrically measured by Ostwald in 1887. Later in 1908, Henderson proposed the equation for the measurement of the concentration of the hydrogen ions by applying the law of mass action. In 1909, Sorensen suggested the pH term. In 1916 Hasselbalch proposed the logarithmic form for the pH. Then in 1923, Bronsted and Lowry proposed the definitions for the acids and bases. Based on this an acid is the donor of the proton and the base is the acceptor of the proton.
ACID-BASE CONCEPT
The following are the concepts that support acid base titrimetry:
- Arrhenius concept: According to the Arrhenius, the definitions for the acids and bases are the substances that produce H+ ions and OH− ions when dissolved in the water.
- Example of acid:
- HCl
H+ + Cl−
- Example of base:
- NaOH
Na+ + OH−
- Examples of Arrhenius acids and bases are as follows:

|
Bases
|
HCl
|
NaOH
|
H2SO4
|
NH4OH
|
CH3COOH
|
KOH
|
HNO3
|
LiOH
|
An Arrhenius acid should have the following properties:
- Sour taste
- Should turn the litmus paper to red colour
- Should have low pH, that is, below 7
An Arrhenius base should have the following properties:
- Bitter taste
- Should have high pH, that is, above 7
- Should turn the litmus paper to blue
Bronsted-Lowry concept: In 1923 Bronsted and Lowry proposed that an acid is the substance that accepts proton and the base is the substance that donates the proton without aqueous media. Examples of Bronsted-Lowry acids and bases are as follows:
|
Bases
|
HCl
|
Cl−
|
HI
|
I−
|
H2SO4
|
HSO4−
|
An acid-base reaction according to the Bronsted-Lowry is as follows:
HCL + NH3  NH4Cl
NH4Cl
Where, proton is transferred from HCl to NH3. Some substances act as both acids and bases. These compounds are called as amphoteric substances.
Example: Water
H2O  H+ + OH−
H+ + OH−
Lewis concept: Lewis proposed that the acid is the substance that forms the covalent bond by accepting an electron pair from the other substance and base is defined as the substance that forms the covalent bond by donating the electron pair to the other substance.
Example: BF3 + NH3  NH3BF3
NH3BF3
Where, BF3 accepts the electron pair from the NH3 which donates the proton.
ROLE OF THE SOLVENT
The main limitation of the Arrhenius concept is the use of aqueous system. To overcome this, Albert in 1925 proposed the solvent system theory. This states that every solvent contains the equal proportion of the positive ions commonly known as solvonium ions and negative ions commonly known as solvate ions.
Examples:
|
2NH3
|
2H2O
| |
N2O2
|
Based on this, an acid is defined as the substance or solute, which increases the concentration of the solvonium ions and decreases the solvate ions. In addition, the base is defined as the substance or solute which decreases the concentration of the solvonium ions and increases the solvate ions.
Example: NH4NO3 is a strong acid because it increases the NH4+ ions.
KNH2 is a strong base because it increases the NH2− ions.
HClO4 is a strong acid in water.
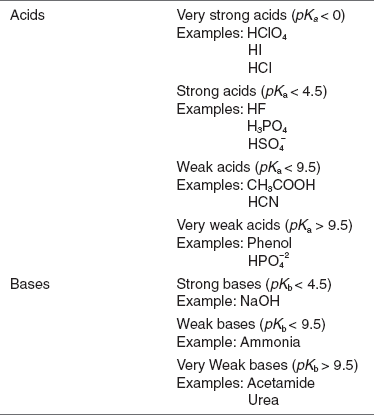
STRENGTHS OF ACIDS AND BASES
The water is completely dissociated into H+ and OH− ions.
H2O  H+ OH−
H+ OH−
The ionic strength of the water is given by the pKw and is given by the following equation:
pKw = [H=][OH−] = 1 × 10−14
From the pH definition which is negative logarithm of the concentration of the [H+] ions,
pH = −log[H+]
Therefore,
pOH = −log[H+][OH−]
From the above equation,
pKw = −log[H+][OH−]
Hence, from the above equations, the strength of acid and base can be calculated. Then consider the normal equation for the acid:
HA  H+ + A−
H+ + A−
The acidity constant or the strength of the acid is given by the pKa.
For the basicity constant consider the following equation:
B + H2O  BH+ + OH−
BH+ + OH−
The basicity constant is given by the pKb and the equation is:
Therefore,
pKw = pKa × pKb = 14
The pKa and pKb values are inversely proportional to the acidity or basicity values of an acid or base. The lower the pKa and pKb values then that much stronger the acid or the base.
Classification of the acids and the bases according to the pKa and pKb values are as follows:
LAW OF MASS ACTION
By applying the law of mass action for the following chemical equation, w get,
xA + yB  K
K  x1C + y1D
x1C + y1D
where A and B are the reactants; C and D are the products; x, y, x1, and y1 are the coefficients for constant quantity which is commonly known as the equilibrium constant (K).
The law of mass action is mainly applied to the acid base titrimetry for the determination of the equilibrium constant.
Solubility Product
Solubility product is defined as the product of the concentration of the ions increased to the appropriate range in a saturated solution at constant temperature. This is mainly expressed for the sparingly soluble salt formed by the acid-base reaction. Consider the following reaction:
Then the solubility product of the salt at constant temperature is given by the following formula:
Ksp = [A+][B+]
This is independent of the salt concentration.
Example: CaSO4  Ca+2 + SO4−2
Ca+2 + SO4−2
Then equilibrium constant is written as the following:
Then it is simplified to get the solubility product of the salt at saturation point at constant temperature.
Ksp = [Ca+2][SO4−2]
In the Ksp expression, the ion concentration is raised to powers equal to molar ratio of the compound.
Common Ion Effect
The addition of the other compound which contains the common ion with salt to the solubility product causes the precipitation of the salt. This is because the Ksp is initially exceeded. The shift in the equilibrium that occurs because of the addition of an ion already present in the equilibrium equation:
AgCl(s)  Ag+ + Cl−
Ag+ + Cl−
The addition of the NaCl solution to this above AgCl shifts the equilibrium position. Here, the common ion is the Cl− which forms the precipitate. The formation of the precipitate is known as the common ion effect. This will be given as:
[Ag+][Cl−]
Hydrolysis of Salt
The formation of the water along with the salt by the reaction of the acid with the base is known as the hydrolysis of salt.
HCl + NaOH  NaCl + H2O
NaCl + H2O
In other theory, when the salt is treated with the water, the equal amounts of the acid and base are formed.
Salt + Water  Acid + Base
Acid + Base
The three types of salts are as follows:
- Neutral salt which is formed by the reaction of the strong acid with the strong base.
- NaOH + HCl
NaCl + H2O
- Acidic salt which is formed by the reaction of the strong acid with the weak base.
- Cu(OH)2 + 2HCl
CuCl2 + 2H2O
- Basic salt which is formed by the reaction of the weak acid with the strong base.
- HF + NaOH
NaF + H2O
Buffer Solutions
Buffer solutions are mainly used to resist the pH change on the addition of small quantity of acid or base. These are mainly composed of weak acid and its conjugate base or weak base and its conjugate acid. The best example of buffer solution is the blood. There are mainly the following two types of buffer solutions:
- Weak acid and its salt:
|
CH3COOH
|
CH3COONa
|
Weak base and its salt:
|
NH4OH
|
NH4ONa
|
Buffers are mainly used to reduce the variation of pH effects. The ability of the buffer to resist the pH change is known as buffer capacity. The buffer capacity can be increased by the increase in the molarity of buffer solution. The buffer capacity is calculated by the following equation:
β = dn/dpH
where dn = number of moles of acid or base added; dpH = pH change.
HANDERSON-HESSELBACH EQUATION
The equation for the determination of the pH is known as the Handerson-Hesselbach equation and is given by the following formula:
Where the H+ ions concentration is given by the following formula:
Where the pH is given by the following formula:
pH = −log[H+]
Therefore,
The above equation is the pH determination for the acid.
The pH determination for the base can also be written as follows:
TYPES OF ACID-BASE TITRATIONS AND TITRATION CURVES
There are mainly four types of titrations in the acid-base titrimetry. They are as follows:
- Strong acid with strong base: The strong acid is completely dissolved in the water and it produces the H+ ions which are neutralized by the OH− ions present in the strong base solution. At the equivalence point, the H+ ions are completely neutralized by the OH− ions. Then the plot is drawn between the pH and volume of the titrant.
- Initially the plot shows the slow rise in the pH and at the end point it shows the sharp rise in the pH. This indicates that the neutralization is complete.
- Example: HCl with NaOH
- Strong acid
strong base curve
- Weak base with strong acid: The weak base is taken into the conical flask and the little quantity of the indicator is added. Then the resulting solution is titrated with the strong acid. The plot between the pH and the volume of the titrant shows the initial decrease in the pH and at the end point it shows the rapid pH drop and remains constant. This indicates the completion of the neutralization by the formation of the salt.
- Example: NH3 with HCl
- Weak base with strong acid
- Weak acid with strong base: The weak acid is partially dissociated into H+ ions that are neutralized by the strong base which produces OH− ions. The weak acid is taken into the conical flask and the appropriate indicator is added and then titrated with the strong base until the colour change persists. Then the plot between the pH value and the volume of the titrant initially shows the low pH and after the addition of the strong base it shows the increase in the pH and at the end point it shows the sharp increase in the pH. This indicates the completion of the neutralization.
- Example: CH3COOH with NaOH
- Weak acid with strong base curve
- Weak acid with weak base: The weak acid is partially dissociated and is neutralized with the NH3 which is a weak base. The weak acid is taken into the conical flask and then the appropriate indicator is added. Then it is titrated with the weak base until the colour change appears. By plotting the pH versus volume of the titrant, the plot shows the continuous decrease in the pH. The pH change is not observed rapidly. The end point is indicated by the formation of the salt.
- Example: CH3COOH with NH3
- Weak acid with weak base curve
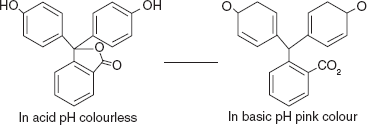
INDICATORS
Acid-base indicator is a weak acid or weak base. This activity is mainly based upon the change in hydrogen ions concentration. The indicator is used to mark the titration end point by change in the colour. The traditional indicator, used in the titrations is litmus.
Example:
Indicator
|
Colour in acid
|
Colour in base
|
Thymol blue
|
Red
|
Yellow
|
Methyl orange
|
Red
|
orange
|
Methyl yellow
|
Red
|
Yellow
|
Bromo phenol blue
|
Yellow
|
Blue
|
Bromo cresol green
|
Yellow
|
Blue
|
Methyl red
|
Red
|
Yellow
|
Phenol red
|
Yellow
|
Red
|
The selection of the indicators for the titration and the change of pH at end point are based upon the pH range of the indicator.
Examples:
|
Titration of HCl with the NaOH with pH change at the end point 3–11 by using methyl orange or phenolphthalein.
|
Titration of acetic acid with the NaOH with pH change at the end point 7–11 by using phenolphthalein.
| |
Titration of HCl with ammonia with the pH change at the end point 3–7 by using methyl orange.
|
Colour formation
The equilibrium of indicators is expressed by the following:
HIn−  H+ + in−
H+ + in−
On the addition of acid we get,
HIn  H+ + ln−
H+ + ln−
Here, hydrogen ion concentration is increased and it produces colour1. On the addition of base, we get,
HIn  H+ + ln−
H+ + ln−
Here, the hydrogen ion concentration is decreased and it produces colour2.
The dissociation constant for the indicator is given by the following equation:
Then the pH is calculated by the following equation:
APPLICATIONS
- Used in the determination of the barbiturates:
- Method: Sample is dissolved in the methanol and water mixture then appropriate indicator for the drug is added and then titrated with the NaOH solution.
- Thymolphthalein is used as indicator for phenobarbital.
- Nizarin yellow is used in case of barbital.
- Methyl orange is used in case of thiopental sodium.
|
For secobarbitol, 0.5g of drug is dissolved in the 10 ml ethanol and 10 ml silver nitrate-pyridine reagent. The resulting solution is titrated with 0.1M ethanolic NaOH until blue colour is attained by using Thymolphthalein as indicator.
|
1ml of 0.1M ethanolic NaOH ≡ 0.026031g of secobarbitol
Used in the determination of the nicotinic acid:
Method: Nicotinic acid is analysed mainly by the titrimetric method based upon the acid-base titration principle. Nicotinic acid is titrated with standard alkali like NaOH using phenolphthalein as indicator.
Used in the amino acids determination:
Method: Sample is added to the 25 ml distilled water and small increments of HCl to maintain the pH to 1.5 and then titrate with the standard NaOH solution using phenolphthalein as indicator. The procedure is continued until the pH is reached to 12.
Used in the periodate assay:
Method: Sample is dissolved in the water and is passed through the cation exchange chamber and the eluates are collected by washing the resin with water. Then 10 ml of the eluate is collected in the conical flask and titrated with the standard sodium hydroxide using phenolphthalein as indicator.
1ml of NaOH ≡ 0.01011 g of iodate
Percentage of iodate = V × N × Equivalent weight factor/sample weight in grams
Used in the determination of aspirin:
Method: Sample is dissolved in the ethanol and then two to three drops of phenolphthalein is added as indicator. Then the resulting solution is titrated with the standard NaOH solution until pink colour is obtained.
Used in the assay of benzoic acid:
Method: Sample is dissolved in the ethanol and water. To this two to three drops of the phenolphthalein is added as indicator then it is titrated with the standard NaOH. Then the percentage of benzoic acid is determined by the following equation:
Percentage of benzoic acid = V × N × Equivalent weight factor/sample weight in grams
REVIEW QUESTIONS
- Give the different definitions for the acids and bases with examples.
- What is Bronsted-Lowry theory?
- Derive the Henderson-Hasselbalch equation.
- Explain about the ionic product of water.
- What is buffer? Add a note on buffer capacity.
- Explain the concept of the acid-base indicators.
- Define pH and list out the factors affecting the pH.
- Explain about the law of mass action.
- What are the different solvents used in acid-base titrimetry?
Comments