Free pharmacy material
Reflection versus scattering: Both reflection and scattering phenomena are very important in turbi-dimetry and nephelometry. If light is allowed to pass through a solution having suspended particles, reflection will take place when the dimensions of suspended particles are larger than the wavelength of incident light. Scattering will take place if the dimensions of suspended particles are smaller than the incident wavelength.

Nephelometry and Turbidimetry
INTRODUCTION
This is mainly used to determine the scattering of the light by the suspended particles present in the sample solution. The instruments used for the measurement of the scattering are called nephelometer and turbidimeters. The choice between the nephelometry and turbidimetry depends upon the fraction of light scattered. This light scattering by the particles which are present in the colloids is known as the Tyndall affect.
Nephelometry is the measurement of the scattered light by the suspended particles at right angles to the incident beam. This method is mainly used for the determination of the low concentration suspensions.
Turbidimetry is the measurement of the transmitted light by the suspended particles to the incident beam. This is used for the determination of the high concentration suspensions.
PRINCIPLE
Light scattering is the physical character of the sample which will depend on the following:
- Particle size
- Wavelength
- Distance of observation
- Concentration of particles
- Molecular weight of particles
The basis of turbidimetric analysis is the measurement of the intensity of transmitted light as a function of the cone of the suspended particles.
In nephelometry, the basic principle involved is the measurement of the intensity of the scattered light as a function of the concentration of the dispensed phase.
Nephelometry and Turbidimetry
|
Reflection versus scattering: Both reflection and scattering phenomena are very important in turbi-dimetry and nephelometry. If light is allowed to pass through a solution having suspended particles, reflection will take place when the dimensions of suspended particles are larger than the wavelength of incident light. Scattering will take place if the dimensions of suspended particles are smaller than the incident wavelength.
FACTORS AFFECTING MEASUREMENT
- Concentration: In turbidimetry, one measures the transmitted of a primary beam of rededication
- T = I/I0
- where I0 is the intensity of incident light; I is the intensity of light after passing through the sample.
- According to Beer's law:
- S = log I0/I = KBC
- where S is the turbidence; B is the path length; K is the proportionality constant; C is the concentration of the sample.
- In nephelometry, the scattered light intensity depends upon a number of factors such as properties of scattering suspension, angle and geometry of the measuring instrument.
- IS = KSI0C
- where KS is the empirical constant; C is the concentration of suspended particles; IS is the scattered of light intensity; I0 is the intensity of incident light.
- Particle geometry: In both turbidimetric and nephelometric analyses, the most critical factor is the control of particle size and shape. One should prepare samples and standards under identical conditions. The conditions include concentration of reactants, temperature, agitation, pH, the presence of non-reactants and order of mixing of reactants.
- Wavelength of incident light: The general practice is to select such a wavelength where the sample solution does not absorb strongly.
- RI difference: Best results are obtained when there is an appreciable RI difference between the particle and its surroundings medium.
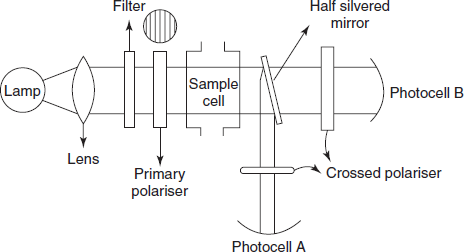
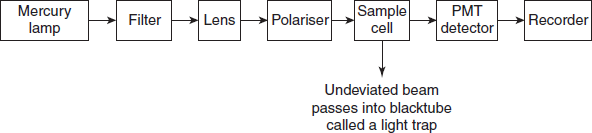
INSTRUMENTATION
Instrumentation used in nephelometry and turbidimetry is very similar to spectrophotometer devices.
- Sources:
- Mercury is lamp: Under light pressure, the excitation of mercury atoms is done by electric discharge.
- Tungsten lamp: It contains a piece of tungsten wire which is heated in a controlled atmosphere.
- Filters: Filters will convert the polychromatic light to monochromatic light. Generally filters are used for this purpose. Fitters are of two types:
- Absorption filters
- Interference filters
- Sample cells: In general, a cell with a rectangular cross-section is preferred, where measurements are to be made at angles other than 90°. Semi-octagonal cells are widely used.
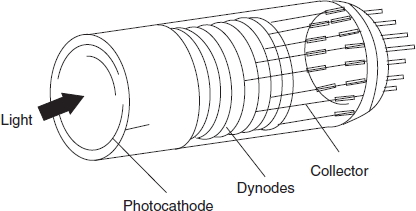
Detectors
Most commonly used detectors in the nephelometry and turbidimetry are photomultiplier tubes. In order to obtain greater sensitivity to very weak light intensities, multiplication of the initial photo-electrons by secondary emission is employed. Several anodes at a gradually increasing potential are contained in one bulb.
Turbidimetres: In most turbidimeters, ordinary calorimeters (or) spectrophotometers may be used.
Nephlometer: The photo-multiplier tube detector is used as a receiver which is mounted on a turnable and may be positioned at any desired angles from 0° to 180° relative to the exit beam.
Sensitivity of the method is improved by the following:
- Addition of water-soluble polymers.
- Gives greater stability of the immune complex.
- Reduced reaction concentrations.
- Increased sensitivity.
APPLICATIONS OF NEPHLOMETRY AND TURBIDIMETRY
- Used in the determination of sulphate as barium sulphate.
- Example: Carbonate as BaCO3
- Chloride as AgCl
- Fluoride as CaF2
- Used in the analysis of water for purity and for the detection of the impurities.
- Used in the determination of CO2.
- Phosphorus can be estimated as a concentration of 1 part in more than 300 million parts of water as a precipitate with strychnine molybdate reagent (mainly used in water treatment plants).
- Used in the determination of turbidity in the sugar products.
- Used in the determination of clarity of citrus juices.
- Used in the determination of benzene percentage in alcohol.
- Used in the determination of amount of amino acids, vitamins and antibiotics.
- Used in the determination of protein.
- Used in the monitoring of the air and water pollution.
- Used in turbidimetric titrations: absorbance versus volume of titrant added is plotted.
- Used in the determination of molecular weight of high polymers:
where H is constant for a given polymer; C is the concentration.
REVIEW QUESTIONS
- What is the main difference in the working principles of nephelometry and turbidimetry?
- What types of compounds are analysed by nephelometry and turbidimetry?
- Explain the working of the nephelometer and turbidimeters.
- What are the main applications of nephelometry and turbidimetry?
Comments