Free pharmacy material





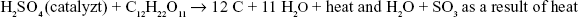






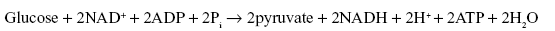

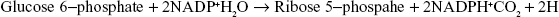

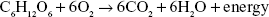



Composition and Metabolism of Carbohydrates
INTRODUCTION to carbohydrates
A carbohydrate is an organic compound with the general formula Cn(H2O)n, that is, consists of only carbon, hydrogen and oxygen, with hydrogen being twice of carbon and oxygen atom ratio. Carbohydrates can be viewed as hydrates of carbon, hence they are known as carbohydrates. Structurally, however, it is more accurate to view them as polyhydroxyaldehydes and ketones.
Carbohydrates are not essential nutrients in humans: the body can obtain all its energy from protein and fats. The brain and neurons generally cannot burn fat for energy, but can use glucose or ketones; the body can also synthesize some glucose from a few of the amino acids in protein and also from the glycerol backbone in triglycerides. Carbohydrate contains 15.8 kilojoules (3.75 kilocalories) and proteins 16.8 kilojoules (4 kilocalories) per gram, while fats contain 37.8 kilojoules (9 kilocalories) per gram. In the case of protein, this is somewhat misleading as only some amino acids are usable as fuel. Likewise, in humans, only some carbohydrates are usable as fuel, as in many monosaccharides and some disaccharides. Other carbohydrate types can be used, but only with the assistance of gut bacteria. Ruminants and termites can even process cellulose, which is indigestible to humans.
Carbohydrates are divided into four chemical groupings:
- Monosaccharides
- Disaccharides
- Oligosaccharides
- Polysaccharides
In general, the monosaccharides and disaccharides, which are smaller carbohydrates, are commonly referred to as sugars. While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, blood sugar is the monosaccharide glucose, table sugar is the disaccharide sucrose, and milk sugar is the disaccharide lactose.
Carbohydrates perform numerous roles in living things. Polysaccharides serve for the storage of energy (e.g. starch and glycogen) and as structural components (e.g. cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g. ATP, FAD, and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA.
3.2 CLASSIFICATION OF CARBOHYDRATES
Carbohydrates are one of the important biomolecules and it is necessary to know their functions and classification. Carbohydrates are classified as monosaccharides, disaccharides, oligosaccharides and polysaccharides. Monosaccharides exhibit isomerism due to the presence of asymmetric carbon atoms. They undergo various reactions to form important derivatives like sugar acids, sugar alcohols, amino sugars and deoxy sugars. Disaccharides can be reducing or non-reducing. Maltose and lactose are reducing sugars.
Sucrose is a non-reducing sugar. Polysaccharides are classified into homopolysaccharides and heteropolysaccharides. Mucopolysaccharides are negatively charged heteropolysaccharides. They are also known as glycosaminoglycans. Glycoproteins mostly contain oligosaccharides which are tightly bound by proteins. In proteoglycans, proteins are covalently bound with mucopolysaccharides.
3.2.1 Monosaccharides
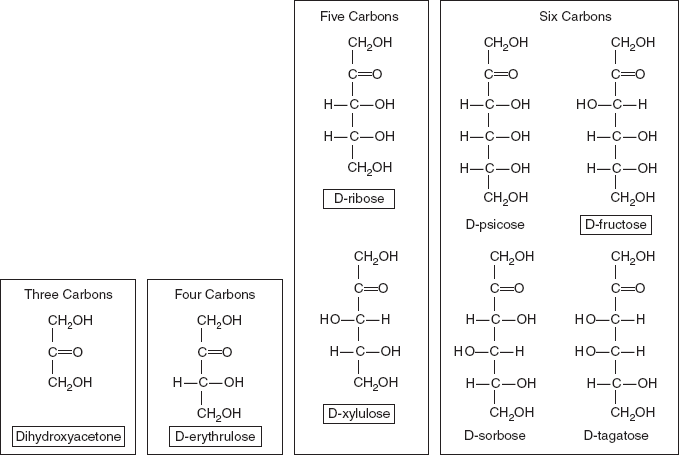
Monosaccharides are the simplest carbohydrates in that they cannot be hydrolysed to smaller carbohydrates. They are aldehydes or ketones with two or more hydroxyl groups. The general chemical formula of an unmodified monosaccharide is (CH2O)n, literally a ‘carbon hydrate’. Monosaccharides are important fuel molecules as well as building blocks for nucleic acids. The smallest monosaccharides, for which n = 3, are dihydroxyacetone and D- and L-glyceraldehyde (Figure 3.1a). The structure of D-glucose and D-fructose are shown in Figure 3.1b. The structure of D-ribose an aldopentose and 2-Deoxy-D-ribose an aldopentose are shown in Figure 3.1c.
Monosaccharides with their names and sources:
Name
|
Source
|
Glucose
|
From Greek word for sweet wine; grape sugar, blood sugar, dextrose.
|
Galactose
|
Greek word for milk–‘galact’, found as a component of lactose in milk.
|
Fructose
|
Latin word for fruit–‘fructus’, also known as levulose, found in fruits and honey; sweetest sugar.
|
Ribose
|
Ribose and deoxyribose are found in the backbone structure of RNA and DNA, respectively.
|
| Monosaccharides |
3.2.2 Disaccharides
Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides, for example, sucrose and lactose. They are composed of two monosaccharide units linked together by a covalent bond known as a glycosidic linkage. The formula of unmodified disaccharides is C12H22O11.
Disaccharides with their names and sources:
Name
|
Source
|
Sucrose
|
French word for sugar–‘sucre’, a disaccharide containing glucose and fructose; table sugar, cane sugar, beet sugar.
|
Lactose
|
Latin word for milk–’lact’; a disaccharide found in milk containing glucose and galactose.
|
Maltose
|
French word for ‘malt’; a disaccharide containing two units of glucose; found in germinating grains, used to make beer.
|
3.2.3 Oligosaccharides
These carbohydrates yield 2–10 monosaccharide units on hydrolysis, for example, maltotriose.
3.2.4 Polysaccharides
Polysaccharides represent an important class of biological polymers. Their function in living organisms is usually either structure or storage-related. Starch (a polymer of glucose) is used as a storage polysaccharide in plants, being found in the form of both amylose and the branched amylopectin. In animals, the structurally similar glucose polymer is the more densely branched glycogen, sometimes called ‘animal starch’. Glycogen's properties allow it to be metabolized more quickly, which suits the active lives of moving animals.
Polysaccharides with their names and sources:
Name
|
Source
|
Starch
|
Plants store glucose as the polysaccharide starch. The cereal grains (wheat, rice, corn, oats, barley) as well as tubers such as potatoes are rich in starch.
|
Cellulose
|
The major component in the rigid cell walls in plants is cellulose and is a linear polysaccharide polymer with many glucose monosaccharide units.
|
Glycogen
|
This is the storage form of glucose in animals and humans which is analogous to the starch in plants. Glycogen is synthesized and stored mainly in the liver and the muscles.
|
3.3 CLASSIFICATION OF MONOSACCHARIDES
Monosaccharides are classified according to three different characteristics: the placement of its carbonyl group, the number of carbon atoms it contains, and its chiral hardness. (A chiral molecule is a type of molecule that lacks an internal plane of symmetry and has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom.)
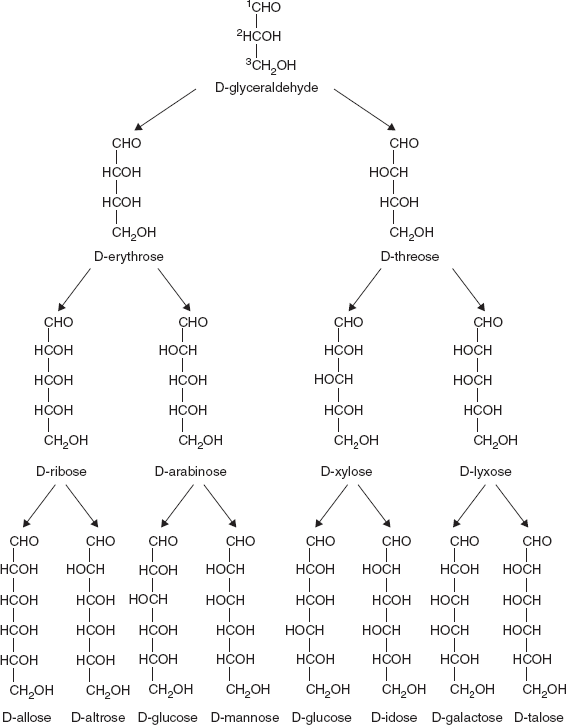
If the carbonyl group is an aldehyde, the monosaccharide is an aldose; if the carbonyl group is a ketone, the monosaccharide is a ketose. Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses, six are hexoses and so on. These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone) (Figures 3.2 and 3.3).
3.3.1 Monosaccharides Have Asymmetric Carbon Atom
A carbon atom to which four different atoms or groups of atom are attached is said to be asymmetric carbon atom (Figure 3.4). All monosaccharides except dihydroxacetone contain one or more asymmetric carbon atoms and thus occur in optically active isomeric form.
3.3.2 Enantiomer
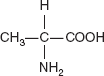
Glyceraldehyde contains one chiral centre and therefore has two different enantiomers. An enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable (not identical), much as one's left and right hands are ‘the same’ but opposite (Figure 3.4).
3.3.3 D and L Forms
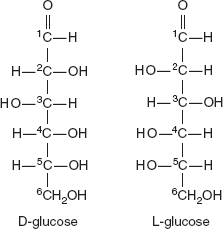
The assignment of D or L is made according to the orientation of the penultimate (last but one) carbon atom from the functional group in a standard Fischer projection; if the hydroxyl group is on the right of the carbon, the sugar is reffered as D otherwise it is an L (Figure 3.5). The ‘D’ and ‘L’ prefixes should not be confused with ‘d’ or ‘l’, which indicate the direction that the sugar rotates plane polarized light. This usage of ‘d’ and ‘l’ is no longer followed in carbohydrate chemistry.
3.3.4 Epimers
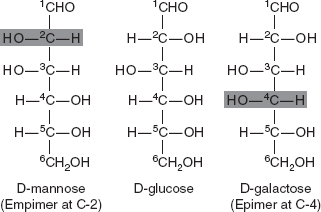
Epimers are diastereomers that differ in configuration of only one stereogenic centre. They are a class of stereoisomers that are non-superposable, non-mirror images of one another. Two sugars that differ only in the configuration around one carbon atom are called epimers. Isomer formed as a result of interchange of the OH and H on carbon atoms C-2, C-3 and C-4 of glucose are known as epimers. Biologically, the most important epimers of glucose are mannose and galactose formed by epimerization at C-2, C-3 and C-4, respectively. In the body, epimerization takes place by the enzyme epimerase (Figure 3.6).
3.3.5 Cyclic Structure of Monosaccharide
The open-chain form of a monosaccharide often coexists with a closed ring form where the aldehyde/ketone carbonyl group carbon (C=O) and hydroxyl group (-OH) react forming a hemiacetal with a new C-O-C bridge.
Figure 3.2 Structure of D-aldoses
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones, respectively. These compounds are formed by formal addition of an alcohol to the carbonyl group (Figure 3.7a and b).
When the alcohol group is replaced by a second alkoxy group, an acetal or a ketal, respectively, is formed (Figure 3.8).
Figure 3.3 D-ketoses
Figure 3.4 Asymmetric Carbon Atom
Figure 3.5 D- and L-glucose
Figure 3.6 Structure of D-mannose, D-glucose and D-galactose
3.3.6 Pyranose and Furanose Rings
Rings with five atoms are called furanose and rings with six atoms are called pyranose forms (Figure 3.9).
A furanose ring structure consists of four carbon and one oxygen atom with the highest numbered chiral carbon (typically to the left of the oxygen in a Haworth projection) determines whether or not the structure has a D-configuration or an L-configuration. In an L-configuration furanose, the substituent on the highest numbered chiral carbon is pointed downwards out of the plane, and in a D-configuration furanose, the highest numbered chiral carbon is facing upwards.
Figure 3.7 (a) Hemiacetal and (b) Hemiketal
Figure 3.8 Cyclic Hemiacetals and Hemiketals
The furanose ring will have either α or β configuration, depending on which direction the anomeric hydroxy group is pointing. In a D-configuration furanose, α configuration has the hydroxy pointing down, and β has the hydroxy pointing up. It is the opposite in an L-configuration furanose (Figure 3.9). Typically, the anomeric carbon undergoes mutarotation in solution, and the α-β configuration switches constantly; thus, it is in an equilibrium state.
Furanoses are less stable than pyranoses as the six-membered ring is more stable.
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six- membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds. A pyranose in which the anomeric OH at C (l) has been converted into an OR group is called a pyranoside.
3.3.7 α and β Anomers
Isomeric forms of monosaccharides that differ only in their configuration about the hemiacetals and hemiketals carbon atom are called anomers. The hemiacetal (or carbonyl) carbon atomis called the anomeric carbon.
The α and β anomers of glucose: the position of the hydroxyl group on the anomeric carbon relative to the CH2OH group bound to carbon 5: the position of hydroxyl group they are either on the opposite sides (α), or the same side (β).
Figure 3.9 Pyran and Furan Rings
In the α anomer, the (-OH) substituent on the anomeric carbon rests on the opposite side (trans) of the ring from the CH2OH side branch.
In the β anomer, in which the CH2OH substituent and the anomeric hydroxyl are on the same side (cis) of the plane of the ring, is called the α anomer (β anomer is cis) (Figure 3.10).
In a Fischer projection, the α anomer is represented with the anomeric hydroxyl group trans to the CH2OH and cis in the β anomer.
The α and β anomers of D glucose interconvert in aqueous solution by a process called mutarotation.
Figure 3.10 Structure of α-D-glucopyranose and β-D-glucopyranose
3.3.8 Isomerism
The subject of isomerism may be divided into structural isomerism and stereoisomerism.
- Structural isomers have the same molecular formula but differ from each other by having different structures.
- Stereoisomers have the same molecular formula and the same structure, but they differ in configuration, that is, in the arrangement of their atoms in space.
3.3.9 Chemical Properties of Monosaccharide
Reducing Sugars
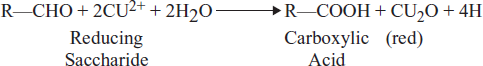
Carbohydrates may be classified as either reducing or non-reducing sugars. The reducing sugars, which are the mcommon, are able to function as reducing agents because free or potentially free aldehyde and ketone groups are present in the molecule. The reducing properties of these carbohydrates are usually observed by their ability to reduce metal ions, notably copper or silver, in alkaline solution.
Benedict's Solution
It is a common reagent for detecting reducing sugars; in this reagent, Cu2+ is maintained in solution as its alkaline citrate complex. When the Cu2+ is reduced, the resulting Cu2+ ion is less soluble and Cu2O precipitates out of the alkaline solution as a yellow or red solid. The reducing sugar in turn is oxidized, fragmented and polymerized in the strongly alkaline Benedict's solution.
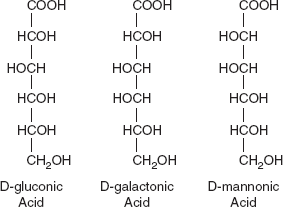
The aldehyde group of aldohexoses is readily oxidized (as shown by its oxidation by Cu2+) to the carboxylic acid at neutral pH by mild oxidizing agents or by enzymes. The monocarboxylic acid that is formed is known as an aldonic acid (e.g. Galactonic acid from galactose). The structure of several of these are shown in Figure 3.11.
Figure 3.11 Structure of D-gluconic Acid, D-galactonic Acid and D-mannonic Acid
Sugar Alcohols
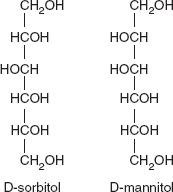
The aldehyde and ketone functions of monosaccharides may be reduced chemically (with hydrogen or NaBH4) or with enzymes to yield the corresponding sugar alcohols. Thus, D-glucose when reduced yields D-sorbitol and D-mannose products D-mannitol. Sorbitol is found in the berries of many higher plants, especially in the Rosaceae; it is crystalline solid at room temperature but has a low melting point. D-mannitol is found in algae and fungi. Both compounds are soluble in H2O and have a sweet taste (Figure 3.12).
Figure 3.12 Structure of D-sorbitol and D-mannitol
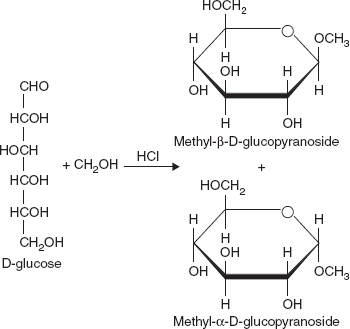
Figure 3.13 Structure of Methyl-β-D-glucopyranoside, and Methyl-α-D-glucopyranoside
Glycoside Formation
One of the more important properties of monosaccharides is their ability to form glycosides or acetals. Consider, as an example, the formation of the methyl glycoside of glucose. When D-glucose in solution is treated with methanol and HCI, two compounds are formed (Figure 3.13).
When an alcoholic hydroxyl group on a second sugar molecule reacts with the hemiacetal (or hemiketal) hydroxyl of another monosaccharide, the resulting glycoside is a disaccharide. The bond between the two sugars is known as a glycosidic bond. Polysaccharides are formed by linking together a large number of monosaccharide units with glycosidic bonds.
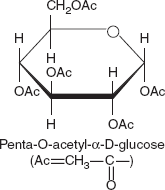
Figure 3.14 Structure of Penta-O-acetyl-α-D-glucose
Ester Formation
When α-D-glucopyranose is treated with acetic anhydride, all the hydroxyl functions are acetylated to yield the penta-O-acetyl glucose shown in Figure 3.14. These acetyl groups, being esters, can be hydrolysed either in acid or alkali.
Oxidation
Oxidation of the carbonyl (aldehyde) carbon of glucose to the carboxyl level produces gluconic acid; other aldoses yield other aldonic acid. Oxidation of the carbon at other end of the carbon chain–C-6 of glucose or galactose or mannose forms uronic acid like glucuronic acid, galacturonic acid or mannuronic acid. As shown here in Figure 3.15 taking Glucose as an example thus forms glucuronic acid.
Figure 3.15 Oxidation
3.4 DISACCHARIDES
Sucrose, also known as table sugar, is a common disaccharide. It is composed of two monosaccharides: D-glucose and D-fructose.
Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage formed via a dehydration reaction, resulting in the loss of a hydrogen atom from one monosaccharide and a hydroxyl group from the other. The formula of unmodified disaccharides is C12H22O11.
3.4.1 Sucrose
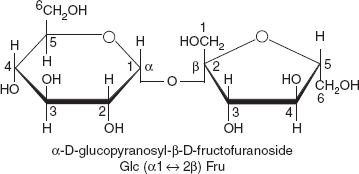
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. This white, odourless, crystalline powder has a pleasing, sweet taste. It is best known for its role in human nutrition. The molecule is a disaccharide derived from glucose and fructose with the molecular formula C12H22O11 (Figure 3.16). The systematic name for sucrose, α-D-glucopyranosyl-(1→2)-D-fructofuranoside, indicates four things:
- Its monosaccharides: glucose and fructose.
- Their ring types: glucose is a pyranose and fructose is a furanose.
- How they are linked together: the oxygen on carbon number 1, C1 of α-D-glucose is linked to the C2 of D-fructose.
- The o-side suffix indicates that the anomeric carbon of both monosaccharides participates in the glycosidic bond.
Figure 3.16 Structure of Sucrose
Physical and Chemical Properties
- It is hydrolysed to glucose and fructose by the enzyme invertase (sucrase) in the alimentary canal. The products of hydrolysis are absorbed.
- Since it is a non-reducing sugar, it does not reduce Fehling's or Benedict's solutions. It cannot reduce Barfoed's solution too.
- It cannot form osazone with phenylhydrazine.
- Sucrose burns with chloric acid, formed by the reaction of sulfuric acid and potassium chlorate:
- Sucrose can be dehydrated with sulfuric acid to form a black, carbon-rich solid, as indicated in the following idealized equation:
Hydrolysis
Hydrolysis breaks the glycosidic bond, converting sucrose into glucose and fructose. Hydrolysis is, however, so slow that solutions of sucrose can sit for years with negligible change. If the enzyme sucrase is added, however, the reaction will proceed rapidly. Hydrolysis can also be accelerated with acids, such as cream of tartar or lemon juice, both weak acids. Similarly, gastric acidity converts sucrose to glucose and fructose during digestion. Fructose has been implicated as a cause or promoter of juvenile obesity and diabetes.
3.4.2 Lactose
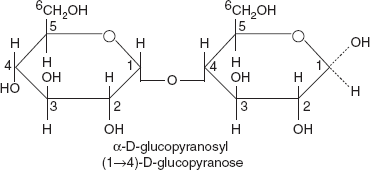
Lactose, a disaccharide composed of one D-galactose molecule and one D-glucose molecule, occurs naturally in mammalian milk (Figure 3.17). The systematic name for lactose is α-D-galactopyranosyl-
(1→4)-D-glucopyranose.
(1→4)-D-glucopyranose.
Lactose is a sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2 ~8 per cent of milk (by weight), although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from lac, the Latin word for milk, plus the -ose ending used to name sugars. It has a formula of C12H22O11.
Figure 3.17 Structure of Lactose
Lactose is a disaccharide that consists of galactose and glucose fragments bonded through β-1→4 glycosidic linkage. Its systematic name is β-D-galactopyranosyl-(1→4)-D-glucose. The glucose fragment can be in either the α-pyranose form or the β-pyranose form, whereas the galactose fragment can only have the β-pyranose form: hence α-lactose and β-lactose refer to anomeric form of the glucopyranose ring alone.
Lactose is hydrolysed to glucose and galactose, isomerized in alkaline solution to lactulose, and catalytically hydrogenated to the corresponding polyhydric alcohol, lactitol.
3.4.3 Maltose
Formed from two units of glucose joined with an α (1→4) bond (Figure 3.18). The isomer isomaltose has two glucose molecules linked through an α (1→6) bond. Maltose is the second member of an important biochemical series of glucose chains. Maltose is the disaccharide produced when amylase breaks down starch. It is found in germinating seeds such as barley as they break down their starch stores to use for food.
Figure 3.18 Structure of Maltose
The addition of another glucose unit yields maltotriose; further additions will produce dextrins (also called maltodextrins) and eventually starch (glucose polymer).
Maltose can be broken down into two glucose molecules by hydrolysis. In living organisms, the enzyme maltase can achieve this very rapidly. In the laboratory, heating with a strong acid for several minutes will produce the same result. Isomaltose is broken by isomaltase.
3.5 POLYSACCHARIDES
Polysaccharides are polymeric carbohydrate structures, formed of repeating units (either mono- or disaccharides) joined together by glycosidic bonds. These structures are often linear, but may contain various degrees of branching. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type the polysaccharide is called a homopolysaccharide, but when more than one type of monosaccharide is present they are called heteropolysaccharides.
For example, storage polysaccharides such as starch and glycogen, and structural polysaccharides such as cellulose and chitin (Figure 3.19).
3.5.1 Storage Polysaccharides Starch
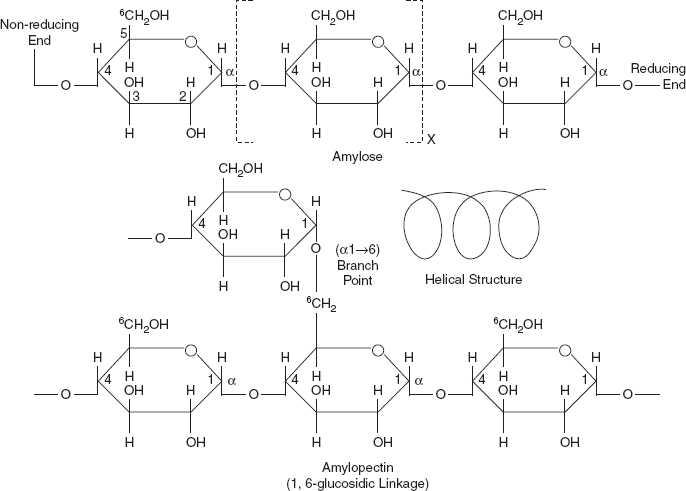
Starch is glucose polymers in which glucopyranose units are bonded by α-linkages. It is made up of a mixture of amylose and amylopectin. Amylose consists of a linear chain of several hundred glucose molecules (Figure 3.20) and amylopectin is a branched molecule made of several thousand glucose units. Both the amylose and amylopectin glucose units are joined by α 1-4 linkages. It is a mixture of two substances—amylose and amylopectin; both are composed of glucopyranose units.
Figure 3.19 Classification of Polysaccharides
Figure 3.20 Structure of Starch
In the amyloses, the glucose units are joined by 1, 4,-α links to form of a helix with six glucose units per turn. Their molecular weights are about 60,000 which are equivalent to about 300–400 glucose units and are responsible for the development of blue colour with iodine. Starch is insoluble in water. They can be digested by hydrolysis, catalyzed by enzymes called amylases, which can break the α-linkages (glycosidic bonds). Humans and other animals have amylazes, so they can digest starches. Potato, rice, wheat and maize are major sources of starch in the human diet. The formation of starches is the way that plants store glucose.
Amylopectin
Amylopectins have much larger molecular weights of about 500,000 and the chains have at least 80 branches, each is at an interval of 24–30 glucose units. The point of branching has alfa 1, 6 linkage. The point of branching is the sixth carbon atom of glucose (Figure 3.20).
Glycogen
Glycogen is a polysaccharide that is found in animals and is composed of a branched chain of glucose residues. It is stored in liver and muscles. It is also formed in plants which do not have chlorophyll system, for example, fungi and yeast. It has a branched structure with straight chain units of 12-18-α-D-glucopyranose [in a 1-4 glucosidic linkage] with branching by means of a [1-6]-glucosidic bonds. It is non-reducing, readily soluble in water and gives a red colour with iodine.
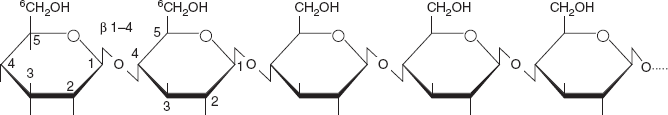
Figure 3.21 Structure of Cellulose
3.5.2 Structural Polysaccharides
Cellulose
The structural components of plants are formed primarily from cellulose. Wood is largely cellulose and lignin, while paper and cotton are nearly pure cellulose. Cellulose is a polymer made with repeated glucose units bonded together by β -1- 4 linkages (Figure 3.21). Humans and many other animals lack an enzyme to break the β-linkages, so they do not digest cellulose. Certain animals can digest cellulose, because bacteria possessing the enzyme are present in their gut. The classic example is the termite. It is made up of β-glucose molecules which are linked by 1:4 linkages.
Chitin
Chitin is one of many naturally occurring polymers. It is one of the most abundant natural materials in the world. Over time it is bio-degradable in the natural environment. Its breakdown may be catalyzed by enzymes called chitinases, secreted by microorganisms such as bacteria and fungi, and produced by some plants. Some of these microorganisms have receptors to simple sugars from the decomposition of chitin. If chitin is detected, they then produce enzymes to digest it by cleaving the glycosidic bonds in order to convert it to simple sugars and ammonia (Figure 3.22).
Figure 3.22 Structure of Chitin
3.6 OLIGOSACCHARIDES
An oligosaccharide is a saccharide polymer containing a small number (typically three to ten) of component sugars, also known as simple sugars (monosaccharides). The name is derived from the Greek word oligos, meaning ‘a few’, and from the Latin/Greek word sacchar which means ‘sugar’. Oligosaccharides can have many functions; for example, they are commonly found on the plasma membrane of animal cells where they can play a role in cell-cell recognition.
They are generally found either O- or N-linked to compatible amino acid side chains in proteins or to lipid moieties.
Oligosaccharides and polysaccharides are composed of longer chains of monosaccharide units bound together by glycosidic bonds. The distinction between the two is based upon the number of monosaccharide units present in the chain. Oligosaccharides typically contain between three and ten monosaccharide units, and polysaccharides contain greater than ten monosaccharide units. Examples of oligosaccharides include the disaccharides, the trisaccharide raffinose and the tetrasaccharide stachyose.
3.7 QUALITATIVE TESTS FOR IDENTIFICATION OF CARBOHYDRATES
3.7.1 Identifying Reducing Sugars
Reducing sugars are oxidized by copper (II) ions in two other saccharide test solutions: Benedict's reagent, a mildly basic solution, and Barfoed's reagent, a mildly acidic solution. The presence of red copper (I) oxide precipitate indicates that the saccharide has reduced the copper (II) ions.
3.7.2 Benedict's Test
Benedict's test is preferred than the Fehling's test for the detection of glucose in urine due to the following causes:
Benedict's test uses a mixture of copper (II) sulphate, sodium citrate and sodium carbonate in a mildly basic solution. This reagent is used as a general test for detecting reducing sugars. If the saccharide is a reducing sugar, it will reduce the copper (II) ions to copper(I) oxide, a red precipitate, as shown in the following equation:
Fehling's solution is composed of copper sulphate sodium potassium tartrate and potassium hydroxide.
- Benedict's test is less likely to give weakly positive results with concentrated urine due to the action of creatinine and uric acid.
- It is more sensitive to small quantities of glucose because of the weaker alkali it contains.
- The strong alkali (KOH) of Fehling's solution can polymerize small quantities of glucose when vigorously boiled.
3.7.3 Barfoed's Test
Barfoed's test uses copper (II) ions in a slightly acidic medium. If the reaction time is carefully monitored, this test can be used to distinguish reducing monosaccharides from reducing disaccharides. Reducing monosaccharides cause the formation of copper (I) oxide within 2–3 minutes. Reducing disaccharides cause the formation of copper (I) oxide after approximately 10 minutes. Equation is shown as follows:
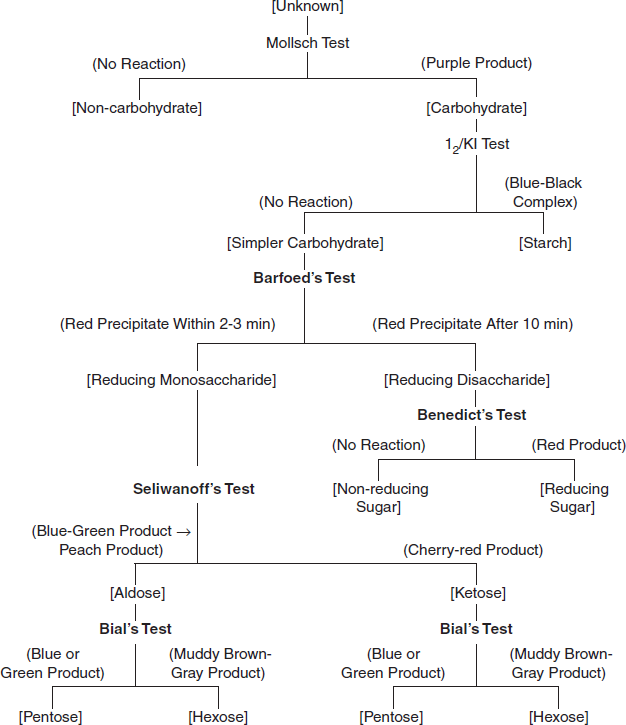
3.7.4 Flowchart for Classifying an Unknown Carbohydrate
To classify the carbohydrates according to the number of molecules contained in the structure and the number of carbon atoms in the carbohydrate unit and also to determine whether the carbohydrate is an aldose or ketose, and whether it is reducing or non-reducing, a classification flowchart is used for carbohydrate identification (Figure 3.23).
Figure 3.23 Flow Chart for Classifying an Unknown Carbohydrate
3.8 INTRODUCTION TO CARBOHYDRATE METABOLISM
There are numerous metabolic pathways, or reactions from beginning to end, which occur in our cells to keep every life process continuing. Anabolic pathways are those which build compounds or synthesize substances for the body, while catabolic pathways result in breaking down compounds. Intermediates are compounds which are formed as pathways proceed. An example of an intermediate in energy production is water—it is recycled through the body as a source of metabolic water. The byproducts of energy production in humans are carbon dioxide gas, water and heat.
Glucose is the key nutrient for most organisms, and it is the central substance in carbohydrate metabolism. During digestion, carbohydrates are hydrolysed to monosaccharides viz. glucose, fructose and galactose, which are absorbed into the bloodstream through the small intestine.
ATP (adenosine triphosphate) is the main energy source for cells, and is the goal of energy production from the nutrients we consume by the oxidation of energy rich compounds. The mitochondria are the major site for ATP production in the cell, and are often referred to as the ‘powerhouse’ of the cell. It is an aerobic cell organelle that is responsible for most energy production in eukaryotic cells. The inner mitochondrial membrane is the site where oxidative phosphorylation occurs. The enzymes of the citric acid cycle are located in the matrix space of the mitochondrion. Anaerobic respiration occurs when no oxygen is present. Aerobic respiration is the oxygen-requiring degradation of energy rich compound and production of ATP, and is the one we shall be concerned with in carbohydrate metabolism. Carbohydrate metabolism has catabolic pathway and anabolic pathway.
The catabolic pathway during carbohydrate metabolism refers to degradation of glycogen to glucose 6- phosphate (glycogenolysis) followed by formation of pyruvate (glycoysis). The pathways from pyruvate to glucose (gluconeogenesis) and from glucose to glycogen constitutes anabolic pathway (glycogenesis).
The functions of metabolism are as follows:
- To release and use energy from foods.
- To synthesize one substance from another.
- To prepare waste products for excretion.
- Vitamins and minerals are ‘keys’ to releasing energy.
3.9 GLYCOLYSIS
Glycolysis is the pathway for the catabolism of glucose that leads to pyruvate. A net of two molecules of ATP per molecule of glucose are produced by substrate level phosphorylation. (Phosphate transfers from organic compounds to ADP, forming ATP). Two molecules of ATP are consumed in the conversion of glucose to fructose-1,6-biphosphate. The first substrate-level phosphorylation of glycolysis is a phosphoryl group transfer from 1,3-biphosphoglycerate to ADP. The second is a phosphoryl group transfer from phosphoenolpyruvate to ADP. NAD+ is also reduced to NADH as glyceraldehyde-3-phosphate is oxidized (Figure 3.24a and 3.24b).
The most common type of glycolysis is the Embden–Meyerhof–Parnas pathway (EMP pathway), which was first discovered by Gustav Embden, Otto Meyerhof and Jakub Karol Parnas.
3.9.1 Reactions of Glycolysis Pathway
In the glycolysis pathway, the breakdown of six-carbon glucose into two molecules of the three-carbon pyruvate takes place in ten steps. The glycolysis pathway consists of two phases: (i) the preparatory phase and (ii) the payoff phase (Figures 3.24(a) and 3.24(b)).
3.9.2 Preparatory Phase of Glycolysis
The first five steps of glycolysis take place in the preparatory phase. In this phase, phosphorylation of glucose and its conversion to glyceraldehydes 3-phosphate takes place. In this phase, two molecules of ATP are invested before cleavage of glucose into three-carbon pieces that is glyceraldehyde three-phosphate and dihydroxyacetone phosphate.
Figure 3.24(a) Preparatoty Phase: Phosphorylation of Glucose and Its Conversion to Glyceraldehyde 3-phosphate
Step 1—First Priming Reaction: D-Glucose is first phosphorylated at the hydroxyl group on C-6. This reaction is catalyzed by the enzyme Hexokinase. This first reaction is endothermic and thus requires energy from a coupled reaction with ATP. ATP is used by being hydrolysed to ADP. In this reaction, there is net loss of one ATP molecule.
Step 2—Isomerization: The glucose-6-phosphate is changed into an isomer, fructose-6-phosphate. This means that the number of atoms is unchanged, but their positions have changed. This reaction is catalyzed by the enzyme Phosphohexose isomerase.
Step 3—Second Priming Reaction: This reaction is virtually identical to step 1. In this step, Fructose-6-phosphate is phosphorylated, but in this reaction phosphate is added to C-1. This reaction is catalyzed by phosphofructokinase.
Figure 3.24(b) Payoff Phase: Oxidative Conversion of Glyceraldehyde 3-phosphate to Pyruvate and the Coupled Formation of ATP and NADH
Again this reaction is endothermic and thus requires energy from a coupled reaction with ATP. ATP is used by being hydrolysed to ADP.
Step 4—Split Molecule in Half: In this step, fructose 1,6-bisphosphate is split into two three-carbon compounds, glyceraldehyde three-phosphate and dihydroxyacetone phosphate. This reaction is catalyzed by the enzyme aldolase.
Step 5—Lysis: In this step, dihydroxyacetone phosphate is isomerized to second molecules of Glyceraldehyde-3-phosphate. This reaction is catalyzed by the enzyme Triose phosphate isomerase. This is the last reaction of the preparatory phase of glycolysis.
Step 6—Payoff Phase of Glycolysis: The second half of the Glycolysis is the payoff phase. The next five steps are the part of the payoff phase. The payoff phase deals with the oxidative conversion of glyceraldehydes 3-phosphate to pyruvate and the coupled formation of ATP and NADH.
Step 7—Oxidation and Phosphorylation: Each molecules of glyceraldehyde-3-phosphate is oxidized and phosphorylated by inorganic phosphate (not by ATP) to form 1,3-bisphosphoglycerate. This reaction is catalyzed by the enzyme glyceraldehyde-3-phosphate dehydrogenase.
This reaction is first an oxidation involving the coenzyme NAD+. The aldehyde is oxidized to an acid as an intermediate through the conversion of NAD+ to NADH + H+. Then, an inorganic phosphate is added in a phosphate ester synthesis.
Step 8—First ATP Forming Reaction (Substrate-level Phosphorylation): In this step, energy is released to molecule of 1, 3-bisphosphoglycerate are converted to 3 phosphoglycerate. This reaction is catalyzed by the enzyme phosphoglycerate kinase. In this step, two molecules of ATP are formed.
Step 9:—In this step, 3-phosphoglycerate is converted to 2-phosphoglycerate. This reaction is catalyzed by the enzyme phosphoglycerate mutase.
Step 10:—In this reaction, 2-phosphoglycerate acid is converted to two molecules of phosphoenolpyruvate. This reaction is catalyzed by the enzyme Enolase with the release of 2 H2O molecules.
Step 11—Second ATP Forming Reaction (Substrate-level Phosphorylation): In this step, two molecules of phosphoenolpyruvate is converted to two molecules of 3-carbon molecule pyruvate (pyruvic acid). This reaction is catalyzed by the enzyme pyruvic kinase. In this step, two molecules of ATP are formed.
Much of this energy is conserved by the coupled phosphorylation of four molecules of ADP to ATP. The net yield is two molecules of ATP per molecule of glucose used, because two molecules of ATP were invested in the preparatory phase. Energy is also conserved in the payoff phase in the formation of two molecules of NADH per molecule of glucose.
In the sequential reactions of glycolysis, three types of chemical transformations are particularly noteworthy: (i) degradation of the carbon skeleton of glucose to yield pyruvate, (ii) phosphorylation of ADP to ATP by high-energy phosphate compounds formed during glycolysis and (iii) transfer of a hydride ion to NAD+-forming NADH.
3.9.3 Fates of Pyruvate After Glycolysis
The pyruvate formed by glycolysis is further metabolized via one of three catabolic routes. In aerobic organism or tissues, under aerobic conditions, glycolysis is only the first stage in the complete degradation of glucose.
The First Route: Pyruvate is oxidized, with loss of its carboxyl group as CO2 to yield the acetyl-coenzyme A; the acetyl group is then oxidized completely to CO2 by the citric acid cycle.
The Second Route: Pyruvate is its reduction to lactate via lactic acid fermentation.
The Third Route: Pyruvate catabolism leads to ethanol.
3.9.4 Overall Process of Glycolysis
If glycolysis were to continue indefinitely, all of the NAD+ would be used up, and glycolysis would stop. To allow glycolysis to continue, organisms must be able to oxidize NADH back to NAD+.
3.9.5 Post-glycolytic Processes
Fermentation
One method of doing this is to simply have the pyruvate do the oxidation; in this process, the pyruvate is converted to lactate in a process called lactic acid fermentation:
This process occurs in the bacteria involved in making yogurt (the lactic acid causes the milk to curdle). This process also occurs in animals under hypoxic (or partially-anaerobic) conditions, found, for example, in overworked muscles that are starved of oxygen, or in infarcted heart muscle cells. In many tissues, this is a cellular last resort for energy; most animal tissue cannot maintain anaerobic respiration for an extended length of time.
Some organisms, such as yeast, convert NADH back to NAD+ in a process called ethanol fermentation. In this process, the pyruvate is converted first to acetaldehyde and carbon dioxide, then to ethanol.
Lactic acid fermentation and ethanol fermentation can occur in the absence of oxygen. This anaerobic fermentation allows many single-cell organisms to use glycolysis as their only energy source.
3.9.6 Anaerobic Respiration
In the above two examples of fermentation, NADH is oxidized by transferring two electrons to pyruvate. However, anaerobic bacteria use a wide variety of compounds as the terminal electron acceptors in cellular respiration: nitrogenous compounds, such as nitrates and nitrites; sulphur compounds, such as sulphates, sulfites, sulphur dioxide and elemental sulphur; carbon dioxide; iron compounds; manganese compounds; cobalt compounds; and uranium compounds.
3.9.7 Aerobic Respiration
In aerobic organisms, a complex mechanism has evolved to use the oxygen in air as the final electron acceptor of respiration.
- First, pyruvate is converted to acetyl-CoA and CO2 within the mitochondria in a process called pyruvate decarboxylation.
- Second, the acetyl-CoA enters the citric acid cycle, or Krebs cycle, where it is fully oxidized to carbon dioxide and water, producing yet more NADH.
- Third, the NADH is oxidized to NAD+ by the electron transport chain, using oxygen as the final electron acceptor. This process creates a ‘hydrogen ion gradient’ across the inner membrane of the mitochondria.
- Fourth, the proton gradient is used to produce a large amount of ATP in a process called oxidative phosphorylation.
3.10 GLUCONEOGENESIS
Gluconeogenesis (abbreviated GNG) is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol and glucogenic amino acids.
It is one of the two main mechanisms humans and many other animals use to keep blood glucose levels from dropping too low (hypoglycemia). The other means of maintaining blood glucose levels is through the degradation of glycogen (glycogenolysis).
Gluconeogenesis is a ubiquitous process, present in plants, animals, fungi, bacteria and other microorganisms. In animals, gluconeogenesis takes place mainly in the liver and, to a lesser extent, in the cortex of kidneys. This process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise and is highly endergonic. Gluconeogenesis is often associated with ketosis. Gluconeogenesis is also a target of therapy for type II diabetes, such as metformin, which inhibits glucose formation and stimulates glucose uptake by cells (Figure 3.25).
Figure 3.25 Gluconeogenesis Pathway With Key Molecules and Enzymes: Steps (1), (3), (7) of Glycolysis Pathway are Irreversible and Cannot be Used in Gluconeogenesis. The Rest of the Steps of Gluconeogenesis are the Same as that of Glycolysis in Reverse Direction
3.10.1 Pathway of Gluconeogenesis
Gluconeogenesis is a pathway consisting of 11 enzyme-catalyzed reactions. The pathway can begin in the mitochondria or cytoplasm, depending on the substrate being used. Many of the reactions are the reversible steps found in glycolysis (Figure 3.25).
- Gluconeogenesis begins in the mitochondria with the formation of oxaloacetate through carboxylation of pyruvate. This reaction also requires one molecule of ATP, and is catalyzed by pyruvate carboxylase. This enzyme is stimulated by high levels of acetyl-CoA (produced in β-oxidation in the liver) and inhibited by high levels of ADP.
- Oxaloacetate is reduced to malate using NADH, a step required for transporting the mitochondria out.
- Oxidation of Malate to oxaloacetate using NAD+.
- Gluconeogenesis occur in the cytoplasm.
- Oxaloacetate is decarboxylated and phosphorylated to produce phosphoenolpyruvateby phosphoenolpyruvate carboxykinase. One molecule of GTP is hydrolysed to GDP during this reaction.
- The next steps in the reaction are the same as reversed glycolysis. However, fructose-1,6-bisphosphatase converts fructose-1,6-bisphosphate to fructose 6-phosphate, requiring one water molecule and releasing one phosphate. This is also the rate limiting step of gluconeogenesis.
- Glucose-6-phosphate is formed from fructose 6-phosphate by phosphoglucoisomerase. Glucose-6-phosphate can be used in other metabolic pathways or dephosphorylated to free glucose. Whereas, free glucose can easily diffuse in and out of the cell, the phosphorylated form (glucose-6-phosphate) is locked in the cell, a mechanism by which intracellular glucose levels are controlled by cells.
- The final reaction of gluconeogenesis, the formation of glucose, occurs in the lumen of the endoplasmic reticulum where glucose-6-phosphate is hydrolysed by glucose-6-phosphatase to produce glucose. Glucose is shuttled into the cytosol by glucose transporters located in the membrane of the endoplasmic reticulum.
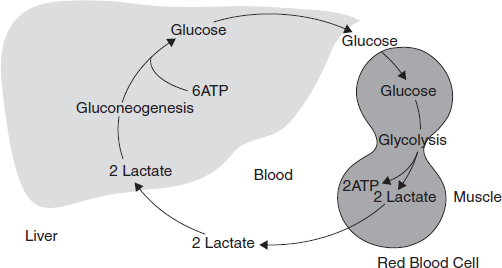
3.11 CORI CYCLE
Muscular activity requires energy, which is provided by the breakdown of glycogen in the skeletal muscles. The breakdown of glycogen, a process known as glycogenolysis, releases glucose in the form of glucose-6-phosphate (G-6-P). G-6-P is readily fed into glycolysis for further oxidation. During muscular activity, the store of ATP needs to be constantly replenished. When the supply of oxygen is sufficient, this energy comes from feeding pyruvate, one product of glycolysis, into the Krebs cycle (Figure 3.26).
Under anaerobic (partial) condition like under intense muscular activity, energy must be released through anaerobic respiration. Anaerobic respiration converts pyruvate to lactate by lactate dehydrogenase. The fermentation step oxidizes the NADH produced by glycolysis back to NAD+, transferring two electrons from NADH to reduce pyruvate into lactate. Most important, fermentation regenerates NAD+, maintaining the NAD+ concentration so that additional glycolysis reactions can occur.
Lactate produced by anaerobic fermentation in muscle cells is transferred to liver. This initiates the other half of the Cori cycle. In the liver, gluconeogenesis reverses both glycolysis and fermentation by converting lactate first into pyruvate, and finally back to glucose. The glucose is then supplied to the muscles through the bloodstream; it is ready to be fed into further glycolysis reactions. If muscle activity has stopped, the glucose is used to replenish the supplies of glycogen through glycogenesis.
Figure 3.26 Cori's Cycle. The Degradation of Glucose and Its Resynthesis During Muscular Activity
3.12 PENTOSE PHOSPHATE PATHWAY
The pentose phosphate pathway is primarily an anabolic pathway that utilizes 6 carbons of glucose to generate 5 carbon sugars and reducing equivalents. However, this pathway oxidizes glucose and under certain conditions can completely oxidize glucose to CO2 and water.
The primary functions of this pathway are as follows:
- To generate reducing equivalents, in the form of NADPH, for reductive biosynthesis reactions that cell need to perform.
- To provide the cell with ribose-5-phosphate (R5P) for the synthesis of the nucleotides and nucleic acids.
- The PPP, can operate to metabolize dietary pentose sugars derived from the digestion of nucleic acids as well as to rearrange the carbon skeletons of dietary carbohydrates into glycolytic/gluconeogenic intermediates.
The cells of the liver, adipose tissue, adrenal cortex, testis and lactating mammary gland have high levels of the PPP enzymes. In fact, 30 per cent of the oxidation of glucose in the liver occurs via the PPP. Additionally, erythrocytes utilize the reactions of the PPP to generate large amounts of NADPH used in the reduction of glutathione (as shown below). The conversion of ribonucleotides to deoxyribonucleotides (through the action of ribonucleotide reductase) requires NADPH as the electron source, therefore, any rapidly proliferating cell needs large quantities of NADPH.
Although the PPP operates in all cells, the highest levels of PPP enzymes (in particular glucose 6-phosphate dehydrogenase) are found in neutrophils and macrophages. These leukocytes are the phagocytic cells of the immune system and they utilize NADPH to generate superoxide radicals from molecular oxygen in a reaction catalyzed by NADPH oxidase.
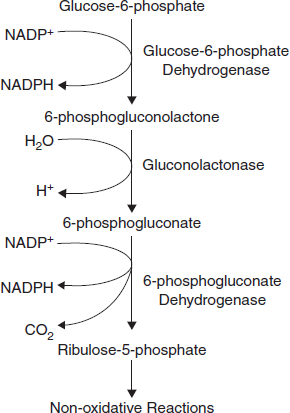
Figure 3.27 Oxidative Stage of Pentose Phosphate Pathway
The oxidation steps, utilizing glucose-6-phosphate (G6P) as the substrate, occur at the beginning of the pathway and are the reactions that generate NADPH. The reactions catalyzed by glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase each generate one mole of NADPH each for every mole of glucose-6-phosphate (G6P) that enters the PPP. The net yield from both of these oxidative reactions is two moles of NADPH per mole of G6P (Figure 3.27).
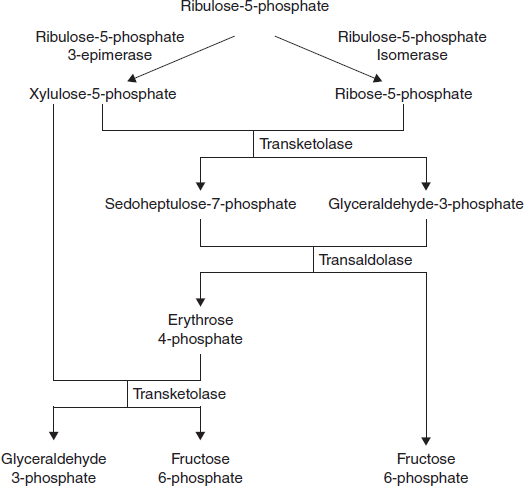
The non-oxidative reactions of the PPP are primarily designed to generate R5P. Equally important reactions of the PPP are to convert dietary 5 carbon sugars into both 6 (fructose-6-phosphate) and 3 (glyceraldehyde-3-phosphate) carbon sugars which can then be utilized by the pathways of glycolysis (Figure 3.28).
The primary enzymes involved in the non-oxidative steps of the PPP are transaldolase and transketolase.
Figure 3.28 Non-oxidative Stage of Pentose Phosphate Pathway
Transketolase functions to transfer two carbon groups from substrates of the PPP, thus rearranging the carbon atoms that enter this pathway. Transketolase requires thiamine pyrophosphate (TPP) as a co-factor in the transfer reaction.
Transaldolase transfers 3 carbon groups and thus is also involved in a rearrangement of the carbon skeletons of the substrates of the PPP.
The net result of the PPP, if not used solely for R5P production, is the oxidation of G6P, a 6 carbon sugar, into a 5 carbon sugar. In turn, 3 moles of 5 carbon sugars are converted, via the enzymes of the PPP, back into two moles of 6 carbon sugars and one mole of 3 carbon sugars. The 6 carbon sugars can be recycled into the pathway in the form of G6P, generating more NADPH. The 3 carbon sugars generated are glyceraldehyde-3-phosphate which can be shunted to glycolysis and oxidized to pyruvate. Alternatively, it can be utilized by the gluconeogenic enzymes to generate more 6 carbon sugars (fructose-6-phosphate or glucose-6-phosphate).
3.13 CITRIC ACID CYCLE
In glycolysis under aerobic conditions, the end product is pyruvic acid. The next step is the formation of acetyl coenzyme A (acetyl CoA) which is the initiator of the citric acid cycle. In carbohydrate metabolism, acetyl CoA is the link between glycolysis and the citric acid cycle.
The citric acid cycle is also known as the Krebs cycle or the tricarboxylic acid cycle. The citric acid cycle contains the final oxidation reactions, coupled to the electron transport chain, which produce the majority of the ATP in the body. The reactions of the citric acid cycle occur in the mitochondria which is also the location of the electron transport chain.
The overall reaction which occurs in the citric acid cycle are as follows:
In the overall scheme of the metabolism of glucose, the citric acid cycle shows where the carbon dioxide comes from and starts the path of hydrogen and electrons into the electron transport chain to produce water and trap energy as ATP (Figure 3.29).
The overall reaction for the metabolism of glucose is written as follows:
3.14 GLYCOGENESIS
Glycogenesis is the process of glycogen synthesis, in which glucose molecules are added to chains of glycogen for storage. This process is activated during rest periods following the Cori cycle, in the liver and also activated by insulin in response to high glucose levels, for example, after a carbohydrate containing meal.
3.14.1 Steps of Glycogenesis Pathway
- Glucose is converted into glu cose-6-phosphate by the action of glucokinase or hexokinase.
- Glucose-6-phosphate is converted into glucose-1-phosphate by the action of Phosphoglucomutase, passing through an obligatory intermediate step of glucose-1,6-bisphosphate.
Figure 3.29 Citric Acid Cycle: Citric Acid Cycle Oxidation Takes Place in Mitochondria
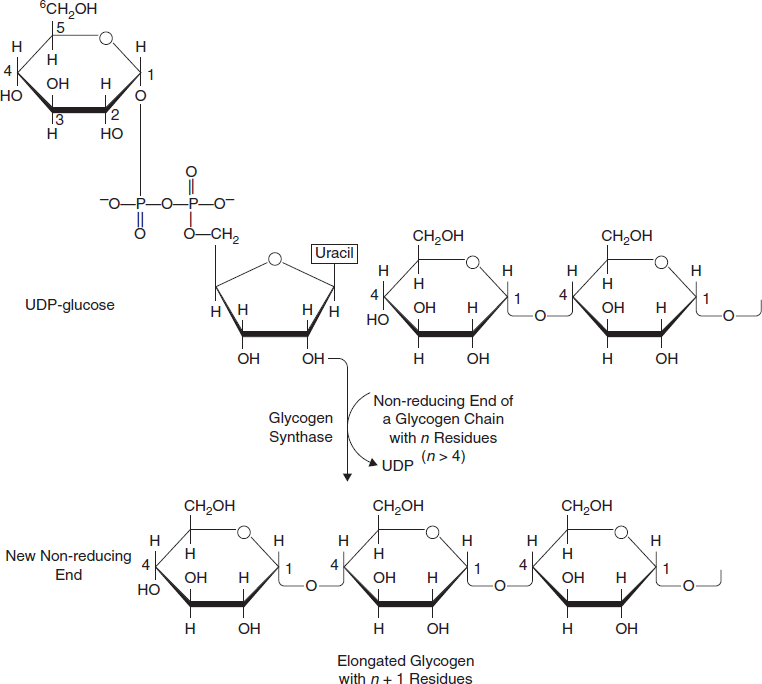
- Glucose-1-phosphate is converted into UDP-glucose (uridine diphosphate glucose) by the action of uridyl transferase (also called UDP-glucose pyrophosphorylase) and pyrophosphate is formed, which is hydrolysed by pyrophosphatase into 2 molecules of Pi.
- Glucose molecules are assembled in a chain by glycogen synthase, which must act on a p glycogenin (glycogen primer or small protein that forms the primer) (Figure 3.30).
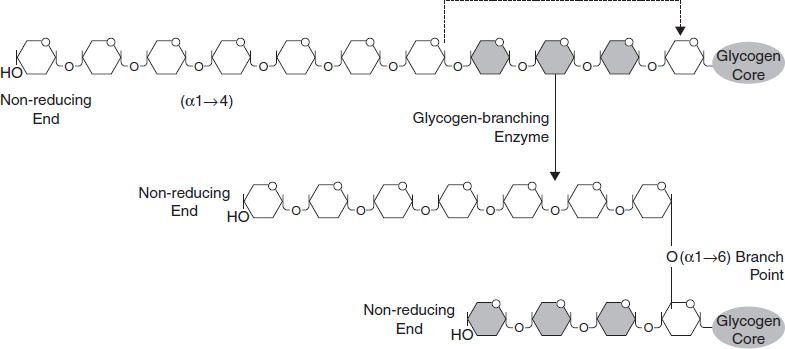
- Branches are made by branching enzyme (also known as amylo- α(1:4)->α (1:6) transglycosylase), which transfers the end of the chain onto an earlier part via α -1:6 glucosidic bond, forming branches, which further grow by addition of more α -1:4 glucosidic units (Figure 3.31).
Figure 3.30 Glycogenesis Pathway Leads to Glycogen Synthesis by Addition of Glucose Mole to the Non-reducing end of a Glycogen Branch to Make a New (α1–4) Linkage With the Help of the Enzyme Glycogen Synthesis
Figure 3.31 Glycogenesis is the Process of Glycogen Synthesis in Which Glucose Molecules are Added to Chains to Form Glycogen for Storage
3.15 GLYCOGENOLYSIS
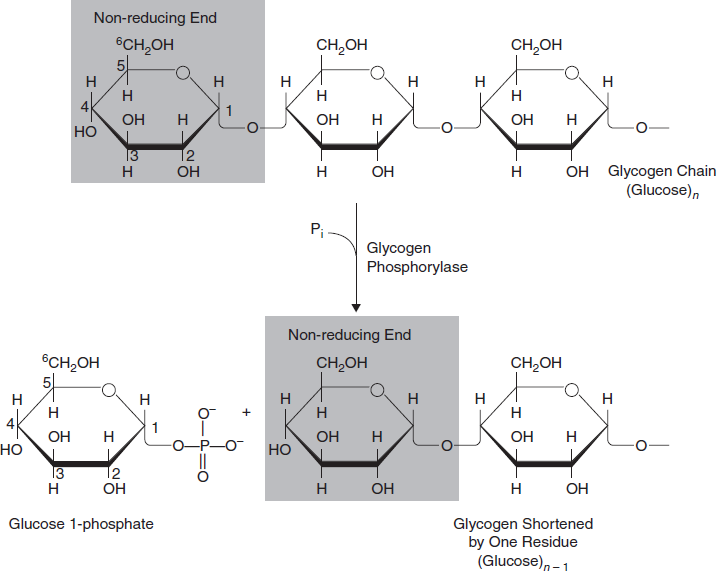
Glycogenolysis (also known as ‘glycogenlysis’) is the conversion of glycogen polymers to glucose monomers. Glycogen is catabolized by removal of a glucose monomer through cleavage with inorganic phosphate to produce glucose-1-phosphate. This derivative of glucose is then converted to glucose-6- phosphate, an intermediate in glycolysis. The hormones glucagon and epinephrine stimulate glycogenolysis.
3.15.1 Function
Glycogenolysis takes place in the muscle and liver tissues, where glycogen is stored, as a hormonal response to epinephrine (e.g., adrenergic stimulation) and/or glucagon, a pancreatic peptide triggered by low blood glucose concentrations produced in the α cells of the islets of Langerhans.
- Liver (hepatic) cells can consume the glucose-6-phosphate in glycolysis, or remove the phosphate group using the enzyme glucose-6-phosphatase and release the free glucose into the bloodstream for uptake by other cells.
- Muscle cells in humans do not possess glucose-6-phosphatase and hence will not release glucose, but instead use the glucose-6-phosphate in glycolysis.
3.15.2 Clinical Significance
Parenteral (intravenous) administration of glucagon is a common human medical intervention in diabetic emergencies when sugar cannot be given orally. It can also be administered intramuscularly.
3.15.3 Reaction
Three steps are involved in glycogenolysis
First step: The overall reaction for the first step is
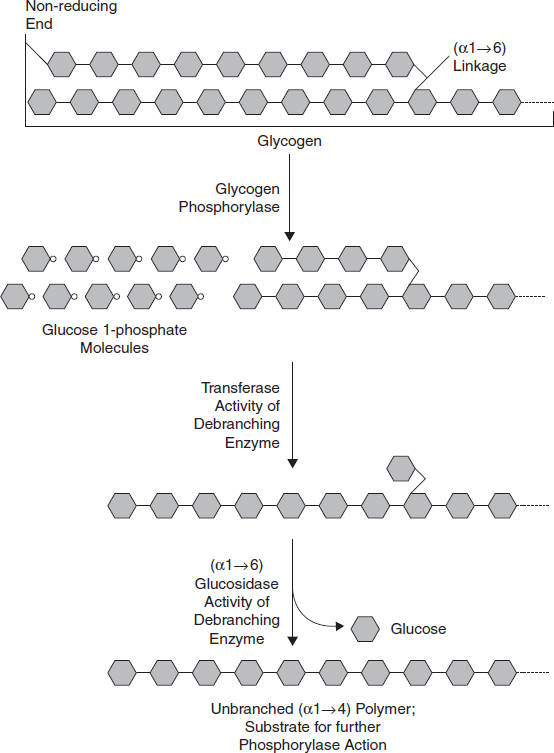
Here, glycogen phosphorylase cleaves the bond at the 1 position by substitution of a phosphoryl group. It breaks down glucose polymer at α -1-4 linkages until 4 linked glucoses are left on the branch (Figure 3.32).
Second step: The second step involves the enzyme [α[1→4]→α[1→4] glucan transferase]/debranching enzyme which transfers the three remaining glucose units to another 1,4 terminal of glycogen; which exposes the α[1→6] branching point. The final action of this enzyme is the hydrolysis of the remaining glucose attached at the α [1→6] branch point which gives one free glucose molecule. This is the only case in which a glycogen metabolite is not a glucose-1-phosphate (Figure 3.33).
Figure 3.32 Glycogenolysis is the Conversion of Glycogen Polymers to Glucose Monomers
Figure 3.33 Glycogen Breakdown Near an (α1–6) Branch Point
Third step: The third and last stage converts G1P (glucose-1-phosphate) to G6P (glucose-6-phosphate) through the enzyme phosphoglucomutase (Figure 3.34).
3.15.4 Regulation
The key regulatory enzyme of the process of glycogenolysis is glycogen phosphorylase:
- Phosphorylation → activation
- Dephosphorylation → inhibition
Figure 3.34 Last Step of Glycogenolysis Which Converts Glucose 1-phosphate to Glucose 6-phosphate by the Enzyme Phosphoglucomutase
3.16 REGULATION OF BLOOD GLUCOSE: GLUCOSE UTILIZATION AFTER A MEAL
A high circulating glucose concentration is present after a meal. Carbohydrate is digested and the glucose absorbed into the blood stream. Insulin is secreted in response.
3.16.1 Insulin
- Stimulates uptake of glucose into both muscle and liver.
- Stimulates increased glycogen synthesis in both muscle and liver.
This is achieved by activation of the key synthesis enzymes. The amount of glycogen which can be stored in these two tissues is limited and once the stores are saturated, excess glucose will be diverted to the synthesis of fats.
3.16.2 Maintenance of Blood Glucose Between Meals
- When there is no dietary glucose intake (between meals), circulating glucose concentration must be maintained.
- The pancreas secretes more glucagon and less insulin.
3.16.3 The Glucagon
- Stops liver glycogen synthesis (by deactivating the synthesis enzymes).
- Increases liver glycogen breakdown (by activating the degradation enzymes).
- Stimulates gluconeogenesis in the liver to further increase the circulating blood glucose concentration. These mechanisms maintain an appropriate circulating blood glucose to supply tissues such as the brain which are major glucose consumers but do not store glycogen.
3.16.4 Supply of Glucose to Exercising Muscle
Increasing muscle activity requires adequate fuel supply for ATP synthesis by muscle. When muscle activity is anticipated, the adrenal glands secrete adrenaline.
Adrenaline increases muscle glycogen degradation (by activating the breakdown enzymes and de-activating the synthesis enzymes).
When muscle activity ceases, adrenaline secretion is switched off. When glucose becomes available again after a meal, glycogen stores in muscle are replenished. Glucose can only be supplied to muscle cells either by utilizing stored muscle glycogen or supply from the liver via the bloodstream.
3.16.5 Glycogen Metabolism in Liver and Muscle
Energy Yield from Glycogen Breakdown
The energy yield from the hydrolysis of stored glycogen and the subsequent oxidation of the released glucose is the same in muscle and liver.
When glycogen is hydrolysed, the product is glucose 1-phosphate. This is easily converted to glucose 6-phosphate. Glucose 6-phosphate is the first product in the glycolysis pathway and its formation from glucose requires the expenditure of 1 ATP molecule/glucose.
As glucose 6-phosphate is formed directly from glycogen hydrolysis, glucose that is derived from glycogen and enters the glycolysis pathway (rather than starting as monomeric glucose) yields a net production of 3 ATP/glucose rather than just 2. This is a 50 per cent increase in yield.
3.16.6 Role of Glucose 6-phosphatase
Muscle and liver have different metabolic needs. Liver supplies other organs with glucose so must be able to export glucose released from glycogen hydrolysis. Muscle is a major consumer of glucose and thus does not export glucose.
Glucose 6-phosphate formed is highly polar and cannot cross the cell's cytoplasmic membrane. To leave the cell it must be converted to glucose. This reaction is catalyzed by an enzyme, glucose 6-phosphatase.
Liver possesses this enzyme, so glucose released from liver glycogen can be exported to other tissues.
It is very important to be aware that muscle does not possess glucose 6-phosphatase, so it does not export glucose released from its glycogen stores, but rather uses it as a fuel to power muscle contraction.
3.16.7 Conversion of Excess Glucose to Fat
Sustained high glucose intake in the diet leads to increased fat synthesis. If glucose intake continues after muscle and liver glycogen stores are saturated, the glucose is not excreted or wasted. It is converted to a fuel storage form which has an unlimited capacity, that is, triglycerides stored in adipose tissue.
Glucose is converted to pyruvate by glycolysis. The pyruvate is converted to acetyl CoA, which is the starting material for the synthesis of fatty acids. This synthesis occurs in the liver followed by conversion of the fatty acids to triglycerides (also in the liver) and then transported to adipose tissue for storage. Triglycerides (fat) form the major energy store in the body.
3.17 CARBOHYDRATE METABOLIC DISORDER AND DISEASES, THEIR INVESTIGATIONS AND INTERPRETATION
3.17.1 Diabetes Mellitus
Primary Idiopathic or Essential Diabetes
Diabetes mellitus means the hyperglycermia due to lack of insulin. This also takes place due to the over-production of other hormones, for example, glucagons, hormones of the anterior pituitary, adrenal and thyroid which are antagonists to insulin or due to increased production of insulinase which inactivates insulin. In India, males are more prone to diabetes mellitus than females.
Treatment of Diabetes Mellitus
- Administration of oral antidiabetic drugs like diabenese—which are not polypeptides—in moderate diabetes and insulin in severe diabetes.
- High protein and low carbohydrate and fat diet are advisable. High protein meal serves as a prolonged source of carbohydrate without rapid hyperglycemic effect and it has protective effect on liver.
- Exercise lowers the blood sugar level stimulating the β-cells of pancreas to liberate more insulin to act on blood sugar for glycogenesis and glycolysis.
- The patient suffering from diabetes mellitus should have normal psychological affairs. The psychological stress and strain stimulate the α cells, of pancreas and adrenal medulla causing the liberation of glucagons and epinephrine, respectively, which have glycogenolytic effect resulting in increased blood sugar (hyperglycemia).
- A diabetic patient requiring more than 200 units/day of insulin is regarded as insulin resistant which is due to antigenic effect of insulin.
3.17.2 Glycogen Storage Diseases
Glycogen storage diseases occur when there is a defect in the enzymes that are involved in the metabolism of glycogen, resulting in growth abnormalities, weakness and confusion (Table 3.1).
- Glycogen storage diseases are caused by lack of an enzyme needed to change glucose into glycogen and break down glycogen into glucose.
- Typical symptoms include weakness, sweating, confusion, kidney stones and stunted growth.
- The diagnosis is made by examining a piece of tissue under a microscope (biopsy).
- Treatment depends on the type of glycogen storage disease and usually involves regulating the intake of carbohydrates.
Table 3.1 Types and Characteristics of Glycogen Storage Diseases
Name
|
Affected Organs, Tissues or Cells
|
Symptoms
|
Type O
|
Liver or muscle
|
Episodes of low blood sugar levels (hypoglycemia) during fasting if the liver is affected
|
von Gierke's disease (Type IA)
|
Liver and kidney
|
Enlarged liver and kidney, slowed growth, very low blood sugar levels and abnormally high levels of acid, fats and uric acid in blood
|
Type IB
|
Liver and white blood cells
|
Same as in von Gierke's disease but may be less severe. Low white blood cell count, recurring infections, and inflammatory bowel disease
|
Pompe's disease(Type II)
|
All organs
|
Enlarged liver and heart and muscle weakness
|
Forbes’ disease(Type III)
|
Liver, muscle and heart
|
Enlarged liver or cirrhosis, low blood sugar levels, muscle damage, heart damage and weak bones in some people
|
Andersen's disease(Type IV)
|
Liver, muscle and most tissues
|
Cirrhosis, muscle damage and delayed growth and development
|
McArdle disease(Type V)
|
Muscle
|
Muscle cramps or weakness during physical activity
|
Hers’ disease(Type VI)
|
Liver
|
Enlarged liver Episodes of low blood sugar during fasting Often no symptoms
|
Tarui's disease(Type VII)
|
Skeletal muscle and red blood cells
|
Muscle cramps during physical activity and red blood cell destruction (hemolysis)
|
Glycogen is made of many glucose molecules linked together. The sugar glucose is the body's main source of energy for the muscles (including the heart) and brain. Any glucose that is not used immediately for energy is held in reserve in the liver, muscles and kidneys in the form of glycogen and released when needed by the body.
Symptoms
Some of these diseases cause few symptoms. Others are fatal. The specific symptoms—age at which symptoms start—and their severity vary considerably among these diseases. For types II, V and VII, the main symptom is usually weakness. For types I, III and VI, symptoms are low levels of sugar in the blood and protrusion of the abdomen (because excess or abnormal glycogen may enlarge the liver). Low levels of sugar in the blood cause weakness, sweating, confusion and sometimes seizures and coma. Other consequences for children may include stunted growth, frequent infections or sores in the mouth and intestines.
Glycogen storage diseases tend to cause uric acid (a waste product) to accumulate in the joints, which can cause gout and in the kidneys, which can cause kidney stones. In type I glycogen storage disease, kidney failure is common in the second decade of life or later.
Diagnosis and Treatment
The specific type of glycogen storage disease is diagnosed by examining a piece of muscle or liver tissue under a microscope (biopsy).
Treatment depends on the type of glycogen storage disease. For most types, eating many small carbohydrate-rich meals every day helps prevent blood sugar levels from dropping. For people who have glycogen storage diseases that produce low blood sugar levels, levels are maintained by giving uncooked cornstarch every 4 to 6 hours around the clock. For others, it is sometimes necessary to give carbohydrate solutions through a stomach tube all night to prevent low blood sugar levels from occurring at night.
3.17.3 Galactosemia
Galactosemia (a high blood level of galactose) is caused by lack of one of the enzymes necessary for metabolizing galactose, a sugar present in lactose (milk sugar). A metabolite that is toxic to the liver and kidneys builds up. The metabolite also damages the lens of the eye, causing cataracts.
- Galactosemia is caused by lack of one of the enzymes needed to metabolize the sugar in milk.
- Symptoms include vomiting, jaundice, diarrhoea and abnormal growth.
- The diagnosis is based on a blood test.
- Even with adequate treatment, affected children still develop mental and physical problems.
- Treatment involves completely eliminating milk and milk products from the diet.
Galactose is a sugar that is present in milk and in some fruits and vegetables. A deficient enzyme or liver dysfunction can alter the metabolism, which can lead to high levels of galactose in the blood (galactosemia). There are different forms of galactosemia, but the most common and the most severe form is referred to as classic galactosemia.
Symptoms
Newborns with galactosemia seem normal at first but, within a few days or weeks, lose their appetite, vomit, become jaundiced, have diarrhoea and stop growing normally. White blood cell function is affected and serious infections can develop. If treatment is delayed, affected children remain short and become intellectually disabled or may die.
Diagnosis
Galactosemia is detectable with a blood test. Before conception, adults with a sibling or child known to have the disorder can be tested to find out whether they carry the gene that causes the disease. If two carriers conceive a child, that child has a 1 in 4 chance of being born with the disease.
Prognosis
If galactosemia is recognized at birth and adequately treated, liver and kidney problems do not develop, and initial mental development is normal. However, even with adequate treatment, children with galactosemia may have a lower intelligence quotient (IQ) than their siblings, and they often have speech problems. Girls often have ovaries that do not function and only a few are able to conceive naturally. Boys, however, have normal testicular function.
Treatment
Galactosemia is treated by completely eliminating milk and milk products—the source of galactose—from an affected child's diet. Galactose is also present in some fruits, vegetables and sea products, such as seaweed. Doctors are not sure whether the small amounts in these foods can cause problems in the long term. People who have the disorder must restrict galactose intake throughout life.
3.17.4 Hereditary Fructose Intolerance
Hereditary fructose intolerance is caused by lack of the enzyme Aldolase B needed to metabolize fructose. Very small amounts of fructose cause low blood sugar and can lead to kidney and liver damage.
In this disorder, the body is missing an enzyme that allows it to use fructose, a sugar present in table sugar (sucrose) and many fruits. As a result, a by-product of fructose accumulates in the body, blocking the formation of glycogen and its conversion to glucose for use as energy. Ingesting more than tiny amounts of fructose or sucrose causes low blood sugar levels (hypoglycemia), with sweating, confusion and sometimes seizures and coma. Children who continue to eat foods containing fructose develop kidney and liver damage, resulting in jaundice, vomiting, mental deterioration, seizures and death. Chronic symptoms include poor eating, failure to thrive, digestive symptoms, liver failure and kidney damage. For most types of this disorder, early diagnosis and dietary restrictions started early in infancy can help prevent these more serious problems.
The diagnosis is made when a chemical examination of a sample of liver tissue determines that the enzyme is missing. Treatment involves excluding fructose (generally present in sweet fruits), sucrose and sorbitol (a sugar substitute) from the diet. Severe attacks of hypoglycemia respond to glucose given by vein. Milder attacks are treated with glucose tablets, which should be carried by anyone who has hereditary fructose intolerance.
3.17.5 Mucopolysaccharidoses
Mucopolysaccharidoses are a group of hereditary disorders in which complex sugar molecules are not broken down normally and accumulate in harmful amounts in the body tissues. The result is a characteristic facial appearance and abnormalities of the bones, eyes, liver and spleen, sometimes accompanied by intellectual disability.
- Mucopolysaccharidoses occur when the body lacks enzymes needed to break down and store complex sugar molecules (mucopolysaccharides).
- Typically, symptoms include short stature, hairiness and stiff finger joints.
- The diagnosis is based on symptoms and a physical examination.
- Although a normal life span is possible, some types cause premature death.
- A bone marrow transplant may help.
Complex sugar molecules called mucopolysaccharides are essential parts of many of the body tissues. In mucopolysaccharidoses, the body lacks enzymes needed to break down and store mucopolysaccharides. As a result, excess mucopolysaccharides enter the blood and are deposited in abnormal locations throughout the body.
During infancy and childhood, short stature, hairiness and abnormal development become noticeable. The face may appear coarse. Some types of mucopolysaccharidoses cause intellectual disability to develop over several years. In some types, vision or hearing may become impaired. The arteries or heart valves can be affected. Finger joints are often stiff.
A doctor usually bases the diagnosis on the symptoms and a physical examination. The presence of a mucopolysaccharidosis in other family members also suggests the diagnosis. Urine tests may help but are sometimes inaccurate. X-rays may show characteristic bone abnormalities. Mucopolysaccharidoses can be diagnosed before birth by using amniocentesis or chorionic villus sampling (see Genetic Disorders Detection: Chorionic Villus Sampling).
Prognosis and Treatment
The prognosis depends on the type of mucopolysaccharidosis. A normal life span is possible. Some types, usually those that affect the heart, cause premature death.
In one type of mucopolysaccharidosis, attempts at replacing the abnormal enzyme have had limited, temporary success. Bone marrow transplantation may help some people. However, death or disability often results, and this treatment remains controversial.
3.17.6 Disorders of Pyruvate Metabolism
Pyruvate metabolism disorders are caused by a lack of the ability to metabolize a substance called pyruvate. These disorders cause a buildup of lactic acid and a variety of neurologic abnormalities.
- A deficiency in any one of the enzymes involved in pyruvate metabolism leads to one of many disorders.
- Symptoms include seizures, intellectual disability, muscle weakness and coordination problems.
- Some of these disorders are fatal.
- Some children are helped by diets that are either high in fat and low in carbohydrates or high in carbohydrates and low in protein.
Pyruvate is a substance that is formed in the processing of carbohydrates and proteins and that serves as an energy source for cells. Problems with pyruvate metabolism can limit a cell's ability to produce energy and allow a buildup of lactic acid, a waste product. Many enzymes are involved in pyruvate metabolism. A hereditary deficiency in any one of these enzymes results in one of a variety of disorders, depending on which enzyme is missing. Symptoms may develop any time between early infancy and late adulthood. Exercise and infections can worsen symptoms, leading to severe lactic acidosis. These disorders are diagnosed by measuring enzyme activity in cells from the liver or skin.
Pyruvate Dehydrogenase Complex Deficiency: This disorder is caused by a lack of a group of enzymes needed to process pyruvate. This deficiency results in a variety of symptoms, ranging from mild to severe. Some newborns with this deficiency have brain malformations. Other children appear normal at birth but develop symptoms, including weak muscles, seizures, poor coordination and a severe balance problem, later in infancy or childhood. Intellectual disability is common. This disorder cannot be cured, but some children are helped by a diet that is high in fat and low in carbohydrates.
Absence of Pyruvate Carboxylase: Pyruvate carboxylase is an enzyme. A lack of this enzyme causes a very rare condition that interferes with or blocks the production of glucose from pyruvate in the body. Lactic acid and ketones build up in the blood. Often, this disease is fatal. Children who survive have seizures and severe intellectual disability, although there are recent reports of children with milder symptoms. There is no cure, but some children are helped by eating frequent carbohydrate-rich meals and restricting dietary protein.
3.18 SUMMARY OF CARBOHYDRATE METABOLISM
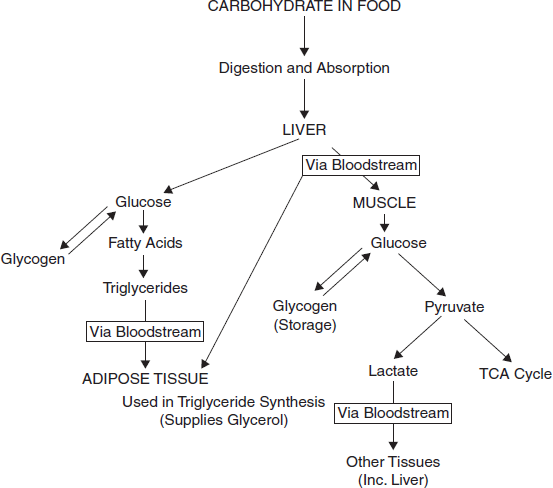
The pathways used in carbohydrate metabolism are shown in img
Overall Carbohydrate Metabolism