Free pharmacy material















General Anaesthetics
INTRODUCTION—
ANAESTHESIA AND ITS STAGES
General anaesthetics are CNS depressants that produce anaesthesia, which extends to the entire body, and are characterized by a state of unconsciousness, analgesia, and amnesia with skeletal muscle relaxation and loss of reflexes.
General anaesthetics are employed for surgical operations, and four stages of anaesthesia may be recognized as:
Stage I (Analgesia): The patient is conscious and experiences sensations of warmth, remoteness, drifting, falling, and giddiness. There is a marked reduction in the perception of painful stimuli. This stage is used often in obstetrics and minor surgery.
Stage II (Delirium): This stage begins with the loss of consciousness. Depression of higher centres produces a variety of effects including excitement, involuntary activity, and increased skeletal muscle tone and respiration.
Stage III (Surgical anaesthesia): This is the stage of unconsciousness and paralysis of reflexes. Respiration is regular and blood pressure is maintained. All surgical procedures are carried out in this stage.
Stage IV (Medullary paralysis): Respiratory and circulatory failures occur as depression of the vital centres of the medulla and brain stem occur.
MECHANISM OF ACTION
The wide variation in structures led to several theories of anaesthetic action. The mechanism by which inhalation anaesthetics manifest their effect is not exactly known. Since they do not belong to one chemical class of compounds, the correlations between structure and activity are also not known. There are a number of hypotheses that have been advanced to explain the action of general anaesthetics; however, none of them can adequately describe the entire spectrum of effects caused by general anaesthetics.
The action of general anaesthetics can be explained as a blockage of ion channels, or as specific changes in mechanisms of the release of neurotransmitters. Three of the proposed mechanisms are discussed here.
- Hydrate hypothesis: Anaesthetic molecules can form hydrates with structured water, which can stop brain function in corresponding areas. However, the correlation between the ability to form hydrates and the activity of inhalation anaesthetics is not known.
- Ion channel hypothesis: Anaesthetics block ion channels by interacting with cellular membranes and reducing the flow of Na+ ions and increasing the flow of K+ ions into the cell, which leads to the development of anaesthesia.
- Fluid membrane hypothesis: Anaesthetics stabilize, or rather immobilize, the cell membrane, hampering membrane fluidity, which produces changes in the ion channel action.
CLASSIFICATION
- Inhalation Anaesthetics
- Liquid: enflurane, isoflurane, halothane, methoxyflurane, ether
- Gas: cyclopropane, nitrous oxide
- Intravenous Anaesthetics
- Ultrashort acting barbiturates: thiopentone, thiamylal, methohexital
- Arylcyclohexylamine: ketamine
- Benzodiazepine: midazolam
- Narcotic analgesics: alfentanil, fentanyl
- Miscellaneous: etomidate, propofol
- Newer Drugs: desflurane, sevoflurane, minaxolone
INHALATION ANAESTHETICS (SYNTHESIS, USES, AND DOSE)
Liquid Anaesthetics
The ideal liquid anaesthetics, yet to be discovered, should have a high margin of safety, produce surgical anaesthesia, have rapid and pleasant induction and recovery, be easily controlled and regulated, have no side-effects or toxicity, should not depress the cardiovascular and respiratory systems, be non-flammable and non-explosive, provide good analgesia and muscle relaxation, and have low cost.
Unfortunately, all available agents exhibit toxic properties that tend to limit their usefulness. Halothane sensitizes the myocardium to sympathoadrenal discharges and adrenaline. Consequently, serious and sometimes fatal cardiac arrhythmias may occur to the patients. Both enflurane and isoflurane are much less likely to sensitize the heart to adrenaline and sympathomimetic discharges. Except for ether, which is not hepatotoxic, all halogenated liquid anaesthetics are capable of producing liver damage. Methoxyflurane is used infrequently because of its low induction and renal toxicity.
Enflurane
2-Chloro-1,1,2-trifluoroethyldifluoromethyl ether
Synthesis
Enflurane is synthesised by chlorinating in light 2-chloro-1,1,2-trifluoroethylmethyl ether to give 2-chloro-1,1,2-trifluoroethyldichloromethyl ether, followed by substitution of chlorine atoms by fluorine on the dichloromethyl group using antimony (III) fluoride.
Uses: Enflurane is a pleasant-smelling, non-flammable, halogenated ether anaesthetic that provides rapid induction with little or no excitement. It provides better analgesia and muscular relaxation than halothane, but high concentrations may cause CVS depression and CNS stimulation.
Dose: Induction: 2.0%–4.5% in oxygen or with oxygen-nitrous oxide mixtures. Induction usually requires 7–10 minutes. Maintenance usually is accomplished with 0.5%–3% concentrations.
Isoflurane
1-Chloro-2,2,2-trifluoroethyl difluoromethyl ether
Synthesis: Isoflurane is synthesised from 2,2,2-trifluoroethanol by methylating with dimethylsulphate. The resulting methyl ether undergoes chlorination by molecular chlorine to give 2-(dichloromethoxy)-1,1,1-trifluoroethane. In the subsequent interaction with hydrogen fluoride in the presence of antimony (V) chloride, chlorine atoms are ultimately replaced by fluorine atoms. The resulting ether again undergoes chlorination by molecular chlorine to give isoflurane.
Uses: Isoflurane, an isomer of enflurane, is a non-flammable inhalation anaesthetic for induction and maintenance of general anaesthesia. Induction of and recovery from isoflurane anaesthesia is rapid. Isoflurane is said to offer advantages over all available inhalation anaesthetics, especially in its lack of any important toxicity.
Dose: Induction: 1.5%–3.0% usually produce surgical anaesthesia in 7–10 minutes. Surgical levels of anaesthesia can be sustained with 1.0%–2.5% concentrations when nitrous oxide is used concomitantly.
Methoxyflurane
2,2-Dichloro-1,1-difluoroethylmethylether
Synthesis
Methoxyflurane is synthesised from 1,1-difluoro-2,2,2-trichloroethane, which undergoes dehydrochlorination by potassium hydroxide to give 1,1-dichloro-2,2-difluoroethylene, to which methanol is added in the presence of potassium hydroxide.
Uses: Methoxyflurane is a potent liquid, volatile anaesthetic agent. A concentration of only 0.1%–2.0% in the inspired mixture will maintain surgical anaesthesia. It provides adequate analgesia and can be used alone in dentistry and obstetrics.
Dose: For analgesia: 0.3%–0.8% in air. For induction: 1.5%–3.0% vaporized by a 1:1 mixture of nitrous oxide and oxygen. For maintenance: 0.1%–2.0%.
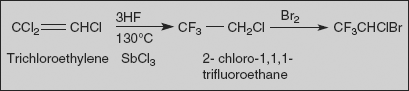
Halothane
2-Bromo-2-chloro-1,1,1- trifluoroethane
Synthesis
Halothane is made by the addition of hydrogen fluoride to tricholoroethylene and simultaneous substitution of chlorine atoms in the presence of antimony (III) chloride at 130°C. The resulting 2-chloro-1,1,1-trifluorethane undergoes further bromination at 450 °C to form halothane.
Uses: Halothane is a potent, relatively safe, frequently employed general inhalation anaesthetic. Induction with halothane is smooth and rapid with little or no excitement. It is not a potent analgesic and skeletal muscle relaxant. Therefore, it is used frequently in conjunction with nitrous oxide and with succinylcholine, tubocurarine, or gallamine.
Dose: For induction: 1.0%–4.0% vaporized by a flow of oxygen or nitrous oxide-oxygen mixture. For maintenance: 0.5%–1.5%.
Ether
Diethyl ether
Synthesis
Ether is prepared by intermolecular dehydration of alcohol. This direct reaction requires drastic conditions (heating to 140 degrees Celsius and an acid catalyst, usually concentrated sulphuric acid).
Use: Ether is an obsolete anaesthetic with a pungent, irritant odour. It is flammable and explosive at concentrations necessary for anaesthesia.
2 Gaseous Anaesthetics
Cyclopropane
Synthesis
Cyclopropane can be prepared in the laboratory by treating 1,3-dichloropropane, zinc dust in aqueous alcohol in the presence of catalytic sodium iodide (Hass cyclopropane process).
Uses: Cyclopropane is an anaesthetic gas with a rapid onset of action. It may be used for analgesia, induction, or maintenance of anaesthesia. Disadvantages include post-anaesthetic nausea, vomiting, headache, and malignant hypertension. In view of these disadvantages, cyclopropane is rarely used.
Nitrous oxide
Synthesis
Nitrous oxide is synthesised either by the thermal decomposition of ammonium nitrate, or by the oxidation of sulphamic acid by nitric acid.
Uses: Nitrous oxide is a weak anaesthetic with good analgesic properties and relatively no skeletal muscle-relaxant properties. Therefore, nitrous oxide is used in conjunction with other liquid anaesthetics. During its administration some patients become hysterical, and because of these characteristics it is often called ‘laughing gas’. Nitrous oxide is used in dental surgery because of the rapid recovery that it allows, and it is employed in obstetrics to produce analgesia.
Dose: Analgesia: 25%–50%; maintenance: 30%–70%. Administered with at least 25%–30% oxygen.
INTRAVENOUS ANAESTHETICS (SYNTHESIS, USES, AND DOSE)
Ultrashort Acting Barbiturates
Rapid acting barbiturates injected most commonly are administered intravenously to induce or sustain surgical anaesthesia. Intravenous anaesthetics are suited best for the induction of anaesthesia and for short procedures, such as orthopaedic manipulations and operations, genito-urinary procedures, obstetric repair, and dilatation and curettage.
Thiopentone sodium
Sodium salt of 5-ethyl-5-(1-methylbutyl)-2-thiobarbiturate
Synthesis
Thiopentone is synthesised from diethylmalonate by alkylating with ethyl bromide in presence of sodium ethoxide to form ethylmalonic ester, which further alkylated with 2-bromopentane in the presence of sodium ethoxide. The product ethyl-(1-methylbutyl)malonic ester undergoes heterocyclization with thiourea, using sodium ethoxide as a base.
Uses: It is the most commonly employed rapidly acting depressant of the CNS, which induces hypnosis and anaesthesia, but not analgesia. It produces anaesthesia within 30–40 seconds after I.V. injection. Recovery after small dose is rapid and it is indicated as the sole anaesthetic agent for brief (15 minutes) procedures, for induction of anaesthesia prior to administration of other anaesthetic agents.
Untoward reactions include respiratory depression, myocardial depression, cardiac arrhythmias, prolonged somnolence and recovery, sneezing, coughing, bronchospasm, larynchospasm, and shivering.
Dose: I.V. induction: 2 ml-3 ml of a 2.5% solution at intervals of 30–60 seconds; maintenance: 0.5 ml–2 ml as required.
Methohexital sodium
Sodium salt of 5-allyl-1-methyl-5-(1-methyl 2-pentynyl) barbiturate
Synthesis
Methohexital is synthesised in the classic manner of making barbituric acid derivatives, in particular by the reaction of malonic ester derivatives with derivatives of urea. The resulting allyl-(1-methyl-2-pentynyl) malonic ester is synthesised by subsequent alkylation of the malonic ester itself, beginning with 2-bromo-3-hexyne, which gives (1-methyl-2-pentynyl) malonic ester, and then by allylbromide. 2-Bromo-3-hexyne is, in turn, synthesised from Normant’s reagent, which is synthesised from 1-butyne and ethylmagnesium bromide and it is subsequent reaction with acetaldehyde followed by bromination of the resulting carbinol using phosphorous tribromide. Interaction of obtained dialkyl malonic ester prepared with N-methylurea gives desired methohexital.
Uses: Uses and untoward effects similar to those of thiopentone.
Dose: I.V. induction: 5 ml–12 ml of a 1% solution at the rate of 1 ml every second; maintenance: 2 ml–4 ml every 4–7 minutes as required.
Thiamylal sodium
Sodium salt of 5-allyl-5-(1-methylbutyl)-2-thiobarbiturate
Synthesis
Thiamylal is synthesised similar to thiopentone sodium, using allyl bromide instead of ethyl bromide in the first step.
Uses: Uses and untoward effects similar to those of thiopentone.
Dose: Induction: 3 ml–6 ml of a 2.5% solution at the rate of 1 ml every 5 seconds; maintenance: 0.5 ml–1 ml of 0.3% solution by continuous drip as required.
Arylcyclohexylamine
Ketamine
2-(2’-Chlorophenyl)-2-(methylamino) cyclohexanone
Synthesis
Ketamine is synthesised from 2-chlorobenzonitrile, which reacts with cyclopentylmagnesium bromide to give 1-(2-chlorobenzoyl)cyclopentane through two step intermediate. The next step is bromination, using bromine to the corresponding bromoketone, which upon interaction with an aqueous solution of methylimino forms the methylimino derivative. During this reaction, a simultaneous hydrolysis of the tertiary bromine atom occurs. On further heating the reaction product in decaline, a ring expansion rearrangement occurs, causing formation of ketamine.
Uses: A rapidly acting non-barbiturate general anaesthetics that produce anaesthesia characterized by profound analgesia. Intravenous doses (2 mg/Kg) produce surgical anaesthesia within 30 seconds and lasts about 10 minutes; intramuscularly (9–13 mg/Kg) produce surgical anaesthesia in 3–4 minutes and last from 12–25 minutes. The clinical anaesthetic state induced by ketamine is termed ‘dissociative anaesthesia’ since the patient may appear awake but is dissociated from the environment and does not respond to pain. Adverse reactions include elevated blood pressure and pulse rate.
Benzodiazepine
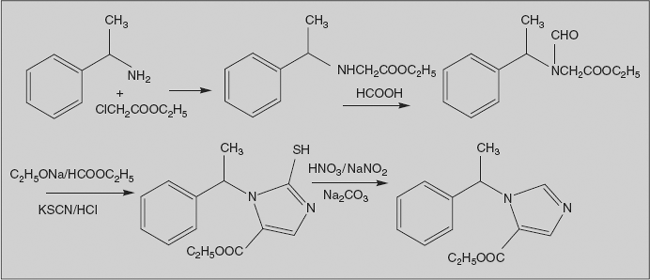
Midazolam
8-Chloro-6 (2’- fluorophenyl) - 2-methyl - imidazo benzodiazepine
Synthesis
Midazolam is prepared from 2-amino-5-chloro-2’-fluoro benzophenone, which undergoes cyclization with ethyl ester of glycine in presence of pyridine to form benzodiazepinone. Amide is converted to thioamide (which is much reactive) by treatment with phosphorouspentasulphide. Reaction of the thioamide with methylamine proceeds to give the amidine; this compound is transformed into a good leaving group by conversion to the N-nitroso derivative by treatment with nitrous acid. Condensation of this intermediate with the carbanion from nitro methane leads to displacement of N-nitroso group by methyl nitro derivative; the double bond shifts into conjugation with the nitro group to afford nitro vinyl derivative. Reduction with Raney nickel followed by reaction with methyl orthoacetate leads to fused imidazoline ring. Dehydrogenation with manganese dioxide converts it into an imidazole to give midazolam.
Uses: Midazolam has been used adjunctively with gaseous anaesthetics. The onset of its CNS effects is slower than that of thiopental, and it has a longer duration of action. Cases of severe post-operative respiratory depression have occurred.
Narcotic Analgesics
Fentanyl and alfentanil are used with other CNS depressants (nitrous oxide, benzodiazepines) in certain high-risk patients who may not survive for a full general anaesthetic.
Fentanyl citrate
N- (1- Phenylethyl - 4- piperidinyl) propionanilide citrate
Synthesis
N-(4-Piperidinyl) aniline is prepared by reductive amination of 4-piperidone and aniline, which condensed with propionyl chloride to form amide. This on N-alkylation with phenyl ethyl chloride affords fentanyl.
Use: Fentanyl citrate relieves moderate to severe breakthrough pain.
Dose: For prompt analgesia during induction, I.V., 0.05 mg–0.1 mg, repeated at 2 to 3 minute intervals until desired effect is achieved; for maintenance I.V., 0.025 mg to 0.05 mg.
Alfentanil
N- [1- [2- (4- Ethyl-4,5- dihydro - 5- oxo - 1H- tetrazol - 1-yl) ethyl] –4-(methoxymethyl)-4- piperidinyl] - N- phenyl propionamide
Synthesis
Alfentanil is synthesised from 1-benzylpiperidine-4-one by means of condensation with aniline in the presence of hydrogen cyanide. The resulting 4-anilino-4-cyano-1-benzylpiperidine undergoes ethanolysis, forming 4-anilino-4-carboethoxy-1-benzylpiperidine, which is reduced by lithium aluminium hydride into 4-anilino-4-hydroxymethyl-1-benzylpiperidine, which is methylated by methyl iodide to give 4-anilino-4-methoxymethyl-1-benzylpiperidine. The resulting product is acylated using propionyl chloride to give 1-benzyl-4-methoxymethyl-4-N-propionyl-anilinopiperidine, which undergoes debenzylation by hydrogen using a palladium on carbon catalyst to give 4-methoxymethyl-4-N-propionylanilinopiperidine, which on reaction with 1-(4-ethyl-4,5-dihydro-5-oxy-1H-tetrazol-1-yl)ethyl-2-chloride gives alfentanil.
Use: It relieves moderate to severe breakthrough pain.
Dose: For 30 minutes anaesthesia, induction: 8–20 µg/Kg; maintenance: 3–5 µg/Kg.
Miscellaneous Drugs
Etomidate
Ethyl - 1- (α- methylbenzyl) imidazole – 5-carboxylate
Synthesis
Etomidate is prepared by the following procedure. The reaction of α-methylbenzylamine with ethyl chloroacetate gives N-ethoxycarbonylmethyl-N-1-phenylethylamine, which undergoes further formylation by formic acid. The resulting N-ethoxycarbonylmethyl-N-formyl-N-1-phenylethylamine undergoes further C-formylation by ethylformate in the presence of sodium ethoxide. The product is further processed by a solution of potassium thiocyanate in hydrochloric acid. As a result of the reaction of thiocyanate ions with the amino group which occurs as a result of acidic hydrolysis of the N-formamide protecting group and further interaction of the obtained intermediate with the newly inserted aldehyde group, a Marckwald reaction-type heterocyclization takes place, resulting in formation of 5-ethoxycarbonyl-2-mercapto-1-(1-phenylethyl)imidazole. Finally, the thiol group is removed by oxidative dethionation upon interaction with a mixture of nitric and nitrous acids (nitric acid in the presence of sodium nitrite), which evidently occurs through formation of unstable sulphinic acid, which easily loses sulphur dioxide resulting in the desired etomidate.
Uses: Intravenous etomidate (0.2–0.6 mg/Kg) produces a rapid induction of anaesthesia with minimal cardiovascular and respiratory changes and without analgesic activity.
Propofol
2,6- Disopropyl phenol
Synthesis
Propofol is prepared by bis-alkylating phenol with isopropyl chloride in the presence of Lewis acid.
Uses: Propofol is similar to the intravenous barbiturates in its rate of onset and duration of anaesthesia. It is used as an induction agent and for short anaesthetic procedures.
5.6 NEWER DRUGS
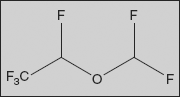
Desflurane
2-(Difluoromethoxy)-1,1,1,2-tetrafluoro-ethane
It is a highly fluorinated methyl ethyl ether used for maintenance of general anaesthesia. Together with sevoflurane, it is gradually replacing isoflurane for human use. It has the most rapid onset and offset of the volatile anaesthetic drugs used for general anaesthesia due to its low solubility in blood.
Sevoflurane
2,2,2-trifluoro-1-[trifluoromethyl]ethyl fluoromethyl ether
It is a sweet-smelling, non-flammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anaesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anaesthesiology. It is often administered in a mixture of nitrous oxide and oxygen. Although desflurane has the lowest blood/gas coefficient of the currently used volatile anaesthetics, sevoflurane is the preferred agent for mask induction due to its lesser irritation to mucous membranes.
Minaxolone
1-(11-(dimethylamino)-2-ethoxy-hexadecahydro-3-hydroxy-10,13-dimethyl-1H-cyclopenta[a]phenanthren-17-yl)ethanone
It is a new water-soluble steroid anaesthetic, and it appears to be a safe and effective intravenous anaesthetic with impressive recovery characteristics. Its only drawback would seem to be its high incidence of excitatory movements and hypertonus. It appears to be a promising intravenous anaesthetic agent worthy of further clinical investigation.