Free pharmacy material





Complexometry
INTRODUCTION
Werner first observed that each atom is surrounded by the maximum number of small which is collectively called as complex. The technique involves the titrating the metal ions with complexing agent which is commonly known as the ligands. The formed coloured complex is used to detect the end point of the titration.
PRINCIPLE
The main principle is the reaction between the ligand and the metal ion to form a complex. The metal ion acts as Lewis acid and the ligand acts as Lewis base which is the complexing agent:
M+2 + Ln  (MLn)+2
(MLn)+2
Examples:
|
Ag+ + 2CN−
|
Cu+2 + 4NH3
| |
Metal ion + chelating agent or complexing agent
|
THEORY
Initially the metal ions are solvated that is, they are dissolved in the appropriate solvent and then these solvent ions are replaced by the ions or other solvent molecules to form the complex. The replacing solvent or ions are known as ligands. The ligands are defined as the charged or neutral species with lone pair of electrons forms the coordinate bond with the metal ions to form complexes.
Examples:
|
Cl−, H2O or NH3 (small molecules);
|
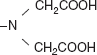
H2NCH2CH2NH2 (ethylene diamine) (large molecules);
| |
EDTA (ethylene diamine tetra acetic acid) (larger molecule).
|
In some conditions, the ligand molecule is able to form a bond with different atoms. These types of ligands are called as ambidentate ligands.
Example: NO2
The following steps play an important role in the complexometric titrations:
- Selection of the complexing agent.
- Detection method employed for the detection of the end point.
- Maintaining of the experimental conditions.
The formation of the complex between the metal and the ligand is obtained by the equilibrium process. The rate of complexation is determined by the equilibrium constant and the dissociation constant.
Example: Cd+2 (aqueous) + 4NH3 (aqueous)  Cd(NH3)+2
Cd(NH3)+2
Then the equilibrium constant Kf is given by the following equation:
Kf = Cd(NH3)+2
Cd+2  NH34
NH34
Then the dissociation constant Kd is given by the following equation:
Kd = Cd+2  NH34 = 1/Kf
NH34 = 1/Kf
Cd(NH3)+2
The above equation can be achieved by the following sets of reactions:
Cd+2 (aqueous) + 4NH3 (aqueous) ↔ Cd(NH3)+2
Cd(NH3)+2 + 4NH3 (aqueous) ↔ Cd(NH3)2+2
Cd(NH3)2+2 + 4NH3 (aqueous) ↔ Cd(NH3)3+2
Cd(NH3)3+2 + 4NH3 (aqueous) ↔ Cd(NH3)4+2
LIGANDS
A ligand is the charged particle or neutral particle which can be readily replaced by the other groups by complex formation (MLn). n is the coordination number of the metal ion and gives the maximum number of ligand groups bound to it.
There are two main classes of ligands as follows:
- Unidentate ligands: Ligands that are bound to a metal ion at one place are called as unidentate ligands.
- Example: NH3
- The following are the steps involved with the ammonia ligand:
- Cd+2 (aqueous) + 4NH3 (aqueous) ↔ Cd(NH3)+2
- Cd(NH3)+2 + 4NH3 (aqueous) ↔ Cd(NH3)2+2
- Cd(NH3)2+2 + 4NH3 (aqueous) ↔ Cd(NH3)3+2
- Cd(NH3)3+2 + 4NH3 (aqueous) ↔ Cd(NH3)4+2
- Bidentate or multidentate ligands: These ligands contain more than one group which is capable of binding with the metal ions.
- Example: ethylene diamine, EDTA, etc.
CHELATING AGENTS
Ligands with more than one electron donating group are called as chelating agents. The ring structure by the ligand groups with the same metal ion is called as chelating agent. The complexing agent itself is called as chelating agent.
Example: EDTA
The chelating agents that form water-soluble complexes with metal ions called as sequestering agents. This removes the metal ion from the solution. The most effective chelating groups in ligand as are amino and carboxylate ions.
Other chelating agents are the following:
DETERMINATION OF THE END POINT
In complexometric titration, the free metal ions are converted to the complex ions. The end point is determined by plotting the PM value which is the negative logarithm of the metal ion concentration versus volume of the titrant. The end point is detected by using an indicator or by applying an instrumental method.
Complexometric titration curve
END POINT DETECTION METHODS
The end point is detected by using visual indicators or by applying instrumental methods.
Indicators: PM indicator is a dye which is capable of forming dye–metal complex. These indicators should posses the following requirements:
- It should be chemically stable.
- The Dye–metal complex formed should be of equal ratio.
- The colour of the indicator should differ from colour of the metal ion.
- It should be selective.
- It should not compete with the EDTA.
- It should be sensitive to the metal ion.
The indicators are classified based on the chemical nature:
- Triphenyl methane dyes.
- Phthalein and substituted phthalates.
- Azo dyes.
- Phenolic compounds.
Examples:
|
Mordant black (red to blue): Ca, Ba, Mg, etc., are detected.
|
Di phenyl carbazone (blue to red).
| |
Alizarin (red to yellow): Pb, Zn, Co, etc., are detected.
| |
Methyl blue (blue to yellow): Pb, Zn, Cd etc., are detected.
| |
Catechol violet (violet to red): Mn, Mg, Fe, etc., are detected.
| |
Xylenol orange (lemon green to yellow): Bi, thorium, etc., are detected.
|
Instrumental methods:
- Spectrophotometric method: In this method, the absorption changes are observed between the metal ion and the complex.
- Potentiometric method: This method is mainly based on the determination of the ions by the specific ion electrodes potential difference. This is calculated by the following equation:
- In this method, saturated calomel is commonly employed.
- Amperometric method: In this method, the mercury electrode is used for the determination of the current developed on a microelectrode at the applied potential.
TYPES OF COMPLEXOMETRIC TITRATIONS
Direct titration: It is simplest and the most convenient method. The standard chelating agent solution is added to the metal ion solution until the end point is detected. In this method, metal ion is added to the suitable buffer solution and appropriate indicator solution and the resulting solution is titrated with the EDTA solution.
Example: Calcium gluconate injection is assayed for determining the calcium chloride.
The main disadvantage are the time consumption of time is more for the complex formation and also the interference of the other ions are observed.
Back titration: In this method, excess of complexing agent is back titrated with the standard solution of the second metal ion. In this method, excess of standard EDTA solution is added to the sample solution and the pH is adjusted. Then the resulting solution is back titrated with the appropriate titrant.
Example: Mn determination and ZnO determination.
Replacement titration: By name itself it indicates the displacement of the metal ion with other metal ion takes place in this method. But it does not give the sharp end points.
Example: Mn+2 + MgEDTA−2  Mg+2 + MnEDTA−2
Mg+2 + MnEDTA−2
Indirect titration: Here protons from the complexing agent are displaced by the heavy metal and titrated with the sodium alkali.
Mn+ + H2X−2  MX(n−4) + 2H+
MX(n−4) + 2H+
Example: This method is used in the analysis of Na, K, Ag, Au, As, Cl, Br and F.
Preparation of the EDTA solution: It is prepared by dissolving accurately weighed EDTA in the distilled water.
Standardization of the EDTA: Accurately weighed granulated zinc is dissolved in the dilute HCl and bromine water by boiling. Then the excess of bromine is removed by boiling and sufficient distilled water is added. The appropriate volume of the above solution is pipetted out and it is neutralized with the sufficient sodium hydroxide solution. Then the solution is diluted with the ammonia buffer and mordant black II solution is added as an indicator. Finally the solution is titrated with the EDTA standard solution.
CONCEPT OF WERNER CO-ORDINATION NUMBER
Werner observed that the atom contains the maximum number of small groups attached to it. This number is called as Werner's co-ordination number. This number depends on the steric factors and valency of the ions.
CONCEPT OF MASKING AND DEMASKING AGENTS
Masking and demasking agents are used for the masking of the reaction of the interfering other metal ions which are called as masking agents and the retaining the ability of the reactants to react are known as the demasking agents. These agents will help to increase the selectivity. The ideal requirements for these agents are the following:
- It should be readily reacted by precipitation.
- It should form stable complexes.
- The colour developed by these agents should not interfere with the end point.
The masking is done either by the precipitation or complexation.
Precipitation: Here the interfering ions are removed by the addition of the precipitating reagents. And these interfering ions are collected as precipitates which are as follows:
Sulphate for Pb and Ba.
Oxalate for Ca and Pb.
Fluoride for Ca, Mg and Pb.
Thioglycerol, etc.
Complexation: Here the interfering ions are removed by the addition of the complexing agents. The complexing agents are as follows:
Ammonium fluoride for Al, Fe and titanium.
Ascorbic acid for iron masking.
Dimercaprol for Hg, Cd Zn As and pb.
KCN for Ag, Cu and Hg.
pH control: The pH control is necessary to increase the stability of the alkaline earth metals such as tin, iron, cobalt and thorium.
FACTORS AFFECTING THE TITRATION VALUES
Nature of the metal ion:
- When the acidity of the metal ion increases, the complex stability also increases.
- Ionic size: The smallest ion forms the stable complex.
- Ionic charge: The higher charge forms the more stable complex is.
Ability of the ligand:
- Basicity of the ligand is directly proportional to the complex stability.
- Size of ligand: The large ligand forms more stable complex.
- Steric effect: Branched ligands form the less stable compounds.
For example, ethylene diamine forms more stable complex than the tetra methyl derivative.
APPLICATIONS
- Used for determining of the hardness of the water.
- Example: Gold ions in ores.
- Method: To the sample, add 1 ml of ammonium hydroxide buffer solution Then solochrome black T is added as indicator and titrated with the standard EDTA solution. Then the total hardness of the water is expressed in the parts per million of the CaCO3.
- Used in the determination of the metal ions.
- Example: Used in the determination of the auric ions.
- Method: This method is carried out by the displacement titration. First, the auric ions are reacted with the tetra Cyano nickelete ions to produce nickelous ions and tetra Cyano aurate ions. Then it is titrated with the standard EDTA solution using eriochrome black T as indicator.
- Au+2 + [Ni(CN)4]−2
[Au(CN)]− + Ni+2
- Used in the preparation of calcium assays.
- Method: In this method, Patton–Reeder's indicator solution is added to the sample which forms the pink calcium ion indicator complex. This solution is then titrated with the standard EDTA solution. The end point is indicated by the formation of blue colour complex. The reaction is as follows:
- Used in the determination of the aluminium hydroxide gel using the hexamine buffer and xylenol orange as indicator and the excess of EDTA is back titrated with the potassium alum.
- Used in the estimation of the magnesium trisilicate by the direct titration method by using the ammonia buffer and mordant black II as indicator.
- Used in the determination of the magnesium sulphate:
- Method: The sample is added to the 1.5 ml of the ammonia buffer solution and then the eriochrome black T is added as indicator. The resulting solution is titrated with the standard EDTA solution until blue colour appeared.
- Used in the determination of the aluminium glycinate:
- Method: The sample is dissolved in the HCl and water mixture by warming. Then excess amount of EDTA solution is added and the pH of the solution is adjusted with standard NaOH. The resulting solution that is excess EDTA is back titrated with the lead nitrate solution using Xylenol orange as indicator.
REVIEW QUESTIONS
- Explain the theory involved in the complex formation.
- What are important steps involved in the complexometry?
- Add a note on ligands and chelating agents.
- Explain the how the endpoint in the complexometry is determined?
- What are the different indicators used in the complexometry?
- What is Werner's co ordination number?
- What are the different types of complexometric titrations?
- Describe the different types of masking and demasking agents.
- Explain the factors affecting the endpoint in the complexometry.
Comments