Free pharmacy material
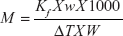

Molecular Weight Determinations
INTRODUCTION
Molecular weight of the compounds is determined by the determination of colligative properties. The methods are as follows:
- Ebullioscopic method
- Cryoscopic method
- Vapour pressure lowering method
- Mass spectrometric method
- Osmometric method
In 1919, Cottrell vapour lift pump made it possible to measure the boiling point which proved useful for the measurement of the molecular weight.
Ebullioscopy
This method is commonly known as the boiling point elevation method. The boiling point is defined as the temperature at which the vapour pressure is equal to the atmospheric pressure. The boiling point depends on the solute dissolved in the solvent. It decreases when a non-volatile solute is present in the solvent. At elevated temperatures, the boiling point is elevated.
ΔT = T – T0
where ΔT = temperature difference between the initial boiling point (T0), which is the boiling point of the pure solvent, and the final boiling point (T), which is the solute boiling point dissolved in the solvent
ΔT = K Δ P
where the ΔP is the vapour pressure lowering.
Then, the molecular weight is determined by the following formula:
ΔT = RTs2 VC/LvM
where ΔT = boiling point elevation; R = gas constant; Ts = boiling point of the solvent; Lv = molar heat of vaporization; C = concentration of the sample solution; M = molecular weight of the compound.
The boiling point is determined by placing the sample and solvent in the boiling point determination apparatus. This consists of the glass vessel with thermometer and condenser.
Cryoscopic Method
This is also known as the freezing point depression method. The freezing point is defined as the temperature at which the solid and liquid phases are at equilibrium. The solute dissolved in the solvent lowers the vapour pressure. The molecular weight is determined by the following equation:
ΔF = RTs2 VC/Lf M
Freezing point depression apparatus
where ΔF = freezing point depression; R = gas constant; Ts = boiling point of the solvent; Lv = molar heat of fusion; C = concentration of the sample solution; M = molecular weight of the compound.
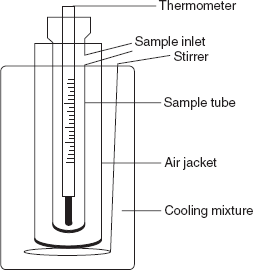
This method was first demonstrated by the Rast method using camphor as a solvent. The apparatus consists of a glass tube with side opening for sample introduction. The glass tube is surrounded by the air jacket and a thermometer is inserted. The glass tube with jacket is inserted into the vessel containing the cooling mixture, that is, salt and ice.
where M = molecular weight; Kf = cryoscopic constant; w = weight of the sample; W = weight of the camphor; ΔT = depression in the freezing point.
Vapour Pressure Lowering
The addition of the sample to the solvent lowers the vapour pressure of the solvent. Then, the molecular weight is determined by the following equation:
ΔP = P0VC/M
where ΔP = vapour pressure lowering; P0 = vapour pressure of the solvent; V = molar volume; C = concentration of the compound; M = molecular weight of the compound.
Osmometry
The basic principle involved in this method is the free diffusion of the solute and solvent molecules through the semi-permeable membrane. A thistle tube with wide opening is stretched with a piece of cellophane which acts as a semi-permeable membrane. The thistle tube is partly filled with the concentrated solution of sucrose and is immersed in the beaker of water. The water is passed through the semi-permeable membrane and creates the osmotic pressure to drive the sucrose solution up to the tube. This process is taken until the both solutions come into equilibrium. This method was first demonstrated by Pfeffer who measured the osmotic pressure of the sugar solutions using the cupric ferrocyanide as the semi-permeable membrane.
Osmometry apparatus
Then the molecular weight is determined by the following equation:
Π = (RT) (C/M)
where Π = osmotic pressure; R = gas constant; T = absolute temperature; C = concentration; M = molecular weight.
Mass Spectrometry
Molecular weight is determined by the mass spectrometer based on their mass–charge ratios.
Mass–charge ratio (m/e) is defined as the charge of sample is divided by the mass of the sample. This is useful for the measurement of the molecular structure and the molecular weight based on the charges on the molecules.
REVIEW QUESTIONS
- Define molecular weight.
- Explain the principle involved in the ebullioscopic method.
- Explain the principle involved in the cryoscopic method.
- Explain the principle involved in the vapour pressure lowering method.
- Explain the principl
Comments