Free pharmacy material


Non-aqueous Titrations
INTRODUCTION
Boyle first recorded the insolubility of the water soluble salts in alcohol. In 1912 Folin and Flanders titrated the acidic substances by using the non-aqueous solvents such as benzene, chloroform and chloroform-methanol mixture. Conant and Hall in 1927 described the behaviour of bases in glacial acetic acid. Lowitz first prepared the moisture-free solvents (non-aqueous solvents). Vorlander first proposed the non-aqueous titration method that is titration of aniline with the HCl in non-aqueous solvent, that is, benzene. The steps involved in the non-aqueous titrimetric method are proposed by the Tomicek. Fritz first used this method to distinguish the aromatic and aliphatic amines by using the perchloric acid as titrant.
PRINCIPLE
The organic acids and bases are insoluble in water. These are extremely weak and cannot be analysed using normal titrimetric methods. Hence the non-aqueous titrimetric method is used. The main principle involved in the non-aqueous titrimetric method is the samples are dissolved in the non-aqueous solvents.
Example: Glacial acetic acid reacts with water which forms oxonium ion with low concentration. To overcome this the glacial acetic acid is dissolved in non-aqueous solvent to form high concentration ions.
Glacial acetic acid reacts with the water and forms oxonium ion with low concentration. The glacial acetic acid dissolved in the non-aqueous solvent forms the high concentration of oxonium ion.
Acid + Non-aqueous solvent  Oxonium ion + Acid anion
Oxonium ion + Acid anion
In Bronsted-Lowry theory, an acid is defined as the substance that donates the proton and the base is defined as the proton acceptor.
HCl  H+ + Cl−
H+ + Cl−
H2O  H+ + H3O+
H+ + H3O+
Then the strength of the acid or base can be measured by the tendency to donate or accept the proton.
The strength of acid is not measured if it is dissolved in the basic solvent. This acidic strength is levelled which is called as levelling effect.
HCl in water acts as strong acid and in glacial acetic acid it acts as weak acid.
THEORY
Water acts as weak acid and weak base.
H2O + H+  H3O+
H3O+
RHH2 + H+  RNH3+
RNH3+
H2O + B  OH− + BH+
OH− + BH+
ROH + B  RO− + BH+
RO− + BH+
In non-aqueous solvents, the acidity decreases in the following order:
HClO4 > HBr > H2SO4 > HCl > HNO3
HCl in water—strongly acidic
HCl in acetic acid—weakly acidic
Acetic acid in water—weakly acidic
Acetic acid in ammonia—strongly acidic
The acids that are titrated by the non-aqueous titration are acid halides, acid anhydrides, carboxylic acids, and amino acids and enols such as xanthenes, imides, phenols, pyrroles, and sulfonamides.
The bases that are titrated by the non-aqueous titration are amines, nitrogen containing heterocyclic compounds, quaternary ammonium compounds, alkali salts of organic acids, and salts of amines.
TYPES OF NON-AQUEOUS SOLVENTS
There are four types of non-aqueous solvents. They are as follows:
- Aprotic solvents: These are chemically neutral substances with low dielectric constants. They are able to react with the acid or base. By the addition of the ionizing solvent the end point is sharpened.
|
Carbon tetra chloride
|
|
Benzene
| |
|
Toluene
|
The picric acid produces a colourless solution in benzene and toluene and produces yellow colour upon the addition of aniline.
Protophilic solvents: These solvents possess high affinity towards the proton. Weak acids are normally used as solutes. A strong protophilic solvent converts the weak acids to strong acids. This mechanism is known as the levelling effect.
|
Liquid ammonia
|
|
Amines
| |
|
Ether
| |
|
Ketones
|
The reaction is as follows:
HA + Non-aqueous solvent ↔ SH+ + A−
Acid + Basic solvent ↔ Solvated proton + Conjugate base of acid
Protogenic solvents: These solvents are acidic in nature and readily donate the proton. These are mainly used to enhance the basicity of weak acid. They show the levelling effect on the bases.
|
Hydrofluoric acid
|
|
Acetic acid
| |
|
Formic acid
| |
|
Sulfuric acid
|
The reaction is as follows:
B + H+ ↔ BH+
Amphiprotic solvents: These solvents combine both properties of protophilic and protogenic solvent properties.
|
Water
|
|
Alcohol
| |
|
Weak acids like acetic acid
|
The reaction is as follows:
CH3COOH ↔ CH3COO− + H+
HClO4 ↔ H+ + ClO4−
CH3COOH + HClO4 ↔ CH3COOH2− + ClO4−
(Onium ion)
When acetic acid dissolved in pyridine which is a basic solvent increases the basicity of the pyridine.
CH3COOH + HClO4 ↔ CH3COOH2+ + ClO4−
C5H5N + CH3COOH ↔ C5H5NH + CH3COO
HClO4 + C5H5N ↔ C5H5NH + ClO4−
In non-aqueous titration, the solvent selection is mainly based upon the following parameters:
- Solubility of the sample
- Nature of the sample
- Should produce sharp end point
- Should have the high dielectric constant
- Should be of low toxicity
- Should be easily purified
- Should be in expensive
Determination of the End Point
The end point in the non-aqueous titrations is determined by the following two methods:
- Potentiometric method: In this method, the end point is determined by using the indicator electrode and reference electrode. Generally, glass electrode is used as the indicator electrode and saturated calomel electrode (SCE) is used as the reference electrode.
- Indicator method: Indicators used in the non-aqueous titrations are as follows:
- Crystal violet: It is used as 0.5% w/v solution in glacial acetic acid. It shows the end point by changing the colour from violet to blue followed by green then to greenish yellow.
- Methyl red: It is used as 0.2% w/v solution in dioxane and changes the colour from yellow to red.
- Naphthol benzein: It is used as 0.2% w/v solution in ethanoic acid and shows the colour changes from yellow to green colour.
- Quinaldein red: It is used as indicator for most of the drug determinations in dimethylformamide and shows the colour changes from purple red to pale green.
- Thymol blue: It is used as 0.2% w/v solution in methanol with colour changes from yellow to blue.
PREPERATION AND STANDARDISATION OF STANDARD SOLUTIONS
- Perchloric acid:
- Preparation of 0.1N perchloric acid: The accurate 8.5 ml of perchloric acid is dissolved in the 100 ml glacial acetic acid and 30 ml of acetic anhydride is added. Then the volume to 1000 ml is made with glacial acetic acid.
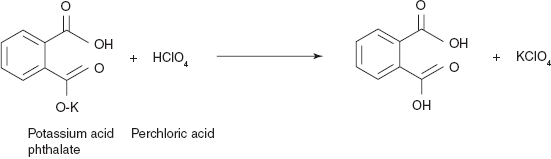
- Standardization of 0.1N perchloric acid: 200 mg of potassium hydrogen phthalate is mixed with the 10 ml of acetic anhydride and the solution is refluxed until the salt is dissolved. Then the solution is cooled to room temperature and little quantity, that is, two to three drops of crystal violet indicator is added. The resulting solution is titrated with the 0.1 N perchloric acid.
- 1 ml of 0.1 N perchloric acid ≡ 0.02041 g of potassium hydrogen phthalate
- Sodium methoxide:
- Preparation of 0.1N sodium methoxide: 2.5 g of the sodium metal is dissolved in the 150 ml of methyl alcohol. Then sufficient volume of the dried toluene is added to make up the volume to 1000 ml.
- Standardization of 0.1N sodium methoxide: 400 mg of the benzoic acid is dissolved in the 80 ml of dimethylformamide and little quantity of thymolphthalein is added as indicator. The resulting solution is titrated with the 0.1 N sodium methoxide until blue colour is obtained.
- 1 ml of 0.1 N sodium methoxide ≡ 0.01221 g of benzoic acid
- Lithium methoxide:
- Preparation of 0.1N lithium methoxide: 700 mg of lithium is mixed with the mixture of solvents in the ratio of 40 ml of methanol and 50 ml of toluene. After dissolving the salt, the volume of the solution is made to 1000 ml with methanol and toluene mixture.
- Standardization of 0.1N lithium methoxide: 0.06 g of benzoic acid is dissolved in the 10 ml of dimethylformamide and thymol blue is added as indicator. The solution is then titrated with the 0.1N lithium methoxide.
- 1 ml of 0.1N lithium methoxide ≡ 0.01221 g of benzoic acid
- Tetrabutylammonium hydroxide:
- Preparation of 0.1N tetrabutylammonium hydroxide: 40 g of tetrabutylammonium hydroxide iodide is dissolved in the 90 ml of methanol. Then 20 g of silver oxide is added to remove iodide present in the solution. The solution is filtered and is made to the volume of 1000 ml by adding it with dry toluene.
- Standardization of 0.1N tetrabutylammonium hydroxide: 60 mg of the benzoic acid is mixed with the 10 ml of dimethylformamide. Then thymol blue solution is added as indicator. Then the solution is titrated with 0.1 N tetrabutylammonium hydroxide.
- 1 ml of 0.1N tetrabutylammonium hydroxide ≡ 0.01221 g of benzoic acid
FACTORS AFFECTING THE NON-AQUEOUS TITRATIONS
The factors affecting the non-aqueous titrations are as follows:
- Acid-base characteristics of the non-aqueous solvents affect the end-point in the non-aqueous titration.
- Examples: In the titration of weak base or acids, the addition of highly acidic or basic solvents increases the acidity or basicity and that increases the consumption of the titrant.
- Protolysis of the substance leads to the increase in the end point.
- The low dielectric constant solvents are commonly employed in the non-aqueous titrations which produce the accurate end points.
Precautions for the non-aqueous titrations are as follows:
- Moisture must be avoided for non-aqueous titrations.
- Carbon dioxide must be avoided for non-aqueous procedures.
DIFFERENT THEORIES OF NON-AQUEOUS TITRATIONS
There are three main theories in the titration of the substances based on the nature by the non-aqueous solvents. They are as follows:
Titration of Weak Bases
The non-aqueous solvents used in the titration of weak bases are of the following two types:
- Neutral solvents:
|
Alcohol
|
|
Chloroform
| |
|
Benzene
|
Acidic solvents:
|
Formic acid
|
|
Glacial acetic acid
|
The titrant commonly employed in the titration of weak bases is perchloric acid. The indicators used in the titration of weak bases are as follows:
- Crystal violet in glacial acetic acid
- Methyl red in glacial acetic acid
- Oracet blue in glacial acetic acid
The procedure is first standardizing the titrant with the suitable solution.
The perchloric acid is standardized with the potassium acid phthalate.
1 ml of perchloric acid = 0.020414 g of potassium acid phthalate
Then perchloric acid in dioxane is standardized.
Examples of weak bases in pharmaceutical compounds are as follows:
- Adrenaline
- Erythromycin
- Metronidazole tartrate
Reaction Mechanism
The examples of drugs and their indicators are as follows:
Drugs (weak bases)
|
Indicators
|
Bisacodyl
|
α–naphtholbenzein
|
Pyrimethamine
|
Quinaldein red
|
Ergometrine
|
Crystal violet
|
Levodopa
|
Oracet blue
|
Metronidazole
|
Brilliant green
|
Titration of the Weak Acids
Many weakly acidic substances are titrated with the non-aqueous method. Solvents used in the titration of weak acids are as follows:
- Ethylenediamine
- n-Butyl amine
The titrants used in the titration of weak acids are as follows:
- Sodium methoxide
- Lithium methoxide
- Potassium methoxide
- Tetrabutylammonium hydroxide
Indicators used in the titration of weak acids are as follows:
- Azo violet
- Thymol blue
- Thymolphthalein
- Nitro aniline
The drugs that contain the weak acids are amino acids and enols.
Titration of Halogen Acid Salts of Bases
Mercuric acetate is added to the halide which replaces the halide ion by equal amount of the acetate ion and the end point is detected by using the crystal violet as indicator.
2RNH2 · HCl ↔ 2RNH3 + Cl−
(CH3COO)2Hg + 2Cl− ↔ HgCl2 + CH3COO−
2CH3COOH2+ + 2CH3COO− ↔ 4CH3COOH
2RNH2 · HCl ↔ 2RNH3 + Cl−
Example: Amitriptyline.HCl assay
2C20H31ON · HCl ↔ C20H31NOH+ + 2Cl−
(CH3COO)2Hg + 2Cl− ↔ HgCl2 + CH3COO−
2CH3COOH2+ + 2CH3COO− ↔ 4CH3COOH
1 ml of perchloric acid ≡ 0.03379 g of amitryptyline.HCl
The examples of drugs and their indicators are as follows:
Drugs (halogen acid salts of bases)
|
Indicators
|
Amantidine HCl
|
Crystal violet
|
Chlorpromazine HCl
|
Methyl orange
|
Cyproheptadine HCl
|
Crystal violet
|
Titration of Amines and Amine Salts of Organic Acids
The primary, secondary and tertiary amines are titrated with the perchloric acid in non-aqueous media like acetic acid. The acetic acid reacts with the weak base B to yield conjugated acid of base BH+ and the conjugated base anion CH3COO−.
B + CH3COOH  BH+ + CH3COO−
BH+ + CH3COO−
Then this anion reacts with the perchloric acid.
HClO4 + CH3COOH ↔ CH3COOH2+ + ClO4−
INTERFERENCE OF THE WATER IN NON-AQUEOUS TITRIMETRY
The drug which is weakly basic or acidic present in the water acts as strong base or acid, which is not able to titrate by the non-aqueous solvent.
ADVANTAGES
- The organic acids and bases that are insoluble in water or in aqueous media are readily analysed by the non-aqueous titrations.
- It is helpful to detect the end point of the sample that is present in the mixture.
- The biological ingredients of the sample are selectively titrated by the non-aqueous titrations.
- These are high accurate methods.
- These produce sharp end points with an internal indicator.
- These are simple and selective.
DISADVANTAGES
- Samples with equal strength to water are cannot be handled by the non-aqueous titrations.
- Aqueous solutions are not handled by the non-aqueous titrations.
- Non-aqueous solvents are not stable compared to aqueous solvents.
- Requires restandardisation of the solvents for every use.
- Temperature corrections are necessary for the non-aqueous solvents.
APPLICATIONS
- Percentage of purity is determined by the assays.
- Example: The sulphonilamide dissolved in 50 ml of dimethylformamide and five drops of thymol blue indicator. Resulting solution is titrated with sodium methoxide and the end point is detected as blue colour.
- where A = milliliters of sodium methoxide; W = weight of the sample; N = Normality of methoxide; EW = equivalent weight factor.
- Used in the determination of the concentration expressions.
- Example: Isoprenaline solutions are mixed with glacial acetic acid and titrate with 0.1N perchloric acid using crystal violet as indicator.
- 1 ml of 0.1 N perchloric acid ≡ 0.5206 g of isoprenaline
- Example: 0.2 g of ethambutol is dissolved in the mixture of 100 ml of acetic acid and 5 ml of mercuric acetate solution and then it is titrated with 0.1 M of perchloric acid (HClO4) using crystal violet as indicator.
- 1 ml of 0.1M HClO4 ≡ 0.01386 g of ethambutol
- Used in the determination of hydrophobic compounds.
- Example: Amantidine HCL Barbiturates alkaloids
- Used in the determination of phenobarbitone.
- Method: Weigh 0.1 g of sample dissolved in 5 ml of pyridine and 0.25 ml of thymolphthalein solution and 10 ml of silver nitrate-pyridine reagent. The resulting solution is titrated with 0.1M ethanolic NaOH until blue colour is attained. Simultaneously a blank is carried out.
- 1 ml of 0.1M ethanolic NaOH ≡ 0.01161 g of phenobarbital.
- Used in the determination of diuretics.
- Example: Small quantity of the drug is dissolved in anhydrous pyridine which is heated and then cooled. The resulting solution is titrated with 0.1M of tetrabutylammonium hydroxide solution.
- 1ml of 0.1M tetrabutylammonium hydroxide ≡ 0.01488gm of hydrochlorothiazide
- Used in the determination of the steroids.
- Example: Methyl sterone, Tetrahydro sterod, Estradiol etc.
- Method: Sample solution is mixed with 2 ml of dimethylformamide and 25 ml of chloroform. 5 ml of resulting solution is taken and then two drops of thymol blue indicator solution is added and titrated with methanolic potassium hydroxide solution. Simultaneously blank is carried out.
- Used in the determination of antitubercular drugs.
- Example: 0.2g of drug is dissolved in the mixture of 100 ml of acetic acid and 5 ml of mercuric acetate solution. Then the resulting solution is titrated with 0.1M perchloric acid (HClO4) using crystal violet as indicator.
- 1ml 0.1M HClO4 ≡ 0.01386g of ethambutol
- Used in the determination of adrenergic drugs.
- Method: Drug solutions are mixed with glacial acetic acid and titrated with 0.1N perchloric acid using crystal violet as indicator.
- 1 ml of 0.1N perchloric acid ≡ 0.5206g of isoprenaline
- ≡ 0.3193g of noradrenaline
- ≡ 0.05767g of salbutamol
- ≡ 28.08g of xylometazoline
REVIEW QUESTIONS
- What is the principle involved in the non-aqueous titrimetry?
- What is main difference between the non-aqueous titrimetry and other methods?
- What are the different types of non-aqueous solvents?
- Explain the principle involved in the titration of weak bases by non-aqueous titrimetry.
- List out the different advantages of non-aqueous titrimetry.
- Explain the procedure involved in the assay of sulphonilamide.
- What are the different factors affecting the non-aqueous titrimetry?
Comments
at feasible prices. our online pharmacy has been working in this business for a long period of time. we understand the needs and requirements of the customers. Join your dots with us today and get this product delivered at your doorstep soon.
https://onlinehealtheducation.com/category/pharmatech/
Dementia.kidney cancer, lung cancer, skin cancer, skin cancer and skin cancer.testicular Cancer, , LEUKEMIA, VIRUSES, HEPATITIS, INFERTILITY WOMEN / MAN, LOT OF LOVE, LOTTERY. ITS CONTACT EMAIL / WHATSAPP: info@drituaherbalcenter.com Or drituaherbalcenter@gmail.com/ +2348149277967
Here is my contact phone number. +1-913-9518-145 if you would need some advise from me.
. Actually, I was looking for the same information on the internet forth7301
and came across your blog. I am impressed by the information that you have on this blog. Thanks once more for designs.
It is a cowardly to say no to herbal medicine. It is fear based. And it is dishonest to what my heart wants. Don't build a wall around yourself because you are afraid of herbals made or taking a bold step especially when it's come to health issues and getting cure. So many young men/ women tell me over and over that Dr Itua is going to scam me but I give him a try to today I feel like no one will ever convince me about herbal medicine I accept Dr Itua herbal medicine because it's cure my herpes just two weeks of drinking it and i have been living for a year and months now I experience outbreak no more, You can contact him if you need his herbal medicine for any such diseases like, Herpes, Schizophrenia,Cancer,Scoliosis,Fibromyalgia,Fluoroquinolone Toxicity Syndrome Fibrodysplasia Ossificans Progressiva.Fatal Familial Insomnia Factor V Leiden Mutation ,Epilepsy Dupuytren's disease,Desmoplastic,Diabetes ,Coeliac disease,Creutzfeldt–Jakob,,Lyme Disease,Epilepsy, ,ALS,Hepatitis,Copd,Parkinson disease.Genetic disease,Fibrodysplasia disease,Fibrodysplasia Ossificans Men/Woman infertility, bowel disease ,Huntington's disease ,Diabetes,Fibroid. disease,Lupus,Lipoid Storage diseases( Gauchers disease),Polycystic Disease.,Cerebral Amyloid Angiopathy, Ataxia,Cirrhosis of Liver,Arthritis,Amyotrophic Lateral Sclerosis,Alzheimer's disease,Adrenocortical carcinoma.Asthma,Allergic,HIV, Epilepsy, Infertility, Love Spell,. Email..drituaherbalcenter@gmail.com then what's app.+2348149277967.... My advice to any sick men/women out there is simple... Be Always an open book. Be gut wrenching honest about yourself, your situation, and what you are all about. Don't hold anything back. Holding back will get you nowhere...maybe a one way ticket to lonelyville and that is NOT somewhere you want to be. So my final truth...and I'm just starting to grasp this one..
Lapox AR-101
and came across your blog. I am impressed by the information that you have on this blog. Thanks a million and please keep up the gratifying work.