Free pharmacy material





Particle Size Analysis
INTRODUCTION
Particle size is the physical property of the compound which influences the stability, solubility and processability. The unit for the particle size is micrometer (μm). There are different methods for the determination of the particle size, as follows:
- Microscopy
- Sieving method
- Sedimentation method
- Sensing zone method
- Light scattering method
- Surface area measurement method
The selection of the method is mainly based upon the following:
- Nature of the compound
- Solubility
- Toxicity
- Flowability
- Cost
- Specifcation requirements, etc.
Types of Diameters
- Martin's diameter: This is the length of the line which bisects the particle.
- Feret's diameter: this is the distance between the two tangents of the particle.
- Projected area diameter: this is the diameter of the circle having the same area as that of the particle.
METHODS FOR PARTICLE SIZE ANALYSIS
Microscopy
The principle of this basic method is to observe the particle size directly by using a microscope. This is helpful in measuring the particle size within a range of 0.2 μm to 100 μm. This method is performed by mounting the sample on a ruled cell and placed for observation. This method is relatively inexpensive, and small sample quantities are required.
This method has the following limitations:
- Time consuming
- It does not give the depth of the particle
- Very low throughput
- No individual particle analysis
To overcome all these disadvantages, advanced methods of the microscopic techniques are used. They include the transmission and electron microscopic methods. These have the following advantages:
- Individual particles are examined by these methods
- Particle shape is measured
- Provides faster analysis
- Is accurate
- Is sensitive
Sieving Method
This method was first used by Arambulo and Deardorff for the particle size analysis in tablet formulations It is based on the grading of different sizes of particles by using different standard sieves. The method involves shaking the sample powder for a standard period of time. For accurate analysis, a set of sieves are arranged in a nest, and the accurately weighed sample is allowed to pass through the sieves. After a definite period of time, the powder retained in each sieve is weighed. This method is applicable for the determination of size ranges from 5 μm to 1mm, which indicates that this method is applicable to wide particle sizes.
The main advantage of this method is that it is inexpensive and easy to perform. The limitations are as follows:
- Lack of reproducibility
- Cleaning is necessary for each analysis
- Requires more people
Based on the sieving method, the powders are classified into three types:
- Coarse powders: 95 per cent powder passes from the no. 1400 sieve
- Moderate fine powders: 95 per cent powder passes from the no. 300 sieve
- Fine powders: 95 per cent powder passes from the no. 180 sieve
Sedimentation Method
The basic principle is that the terminal velocity of the particle in a solution increases by size. This can be achieved by the sedimentation method which is governed by Stoke's law:
where V = rate of settling; dst = mean diameter of the particles; Ps and P0 = densities of the particles and the medium, respectively; g = gravity due to acceleration; η0 = viscosity of the medium.
Therefore,
where h = distance of fall in time.
Anderson pipette apparatus
For the measurement of the particle size by the sedimentation method, the apparatus used is called the Anderson pipette. The procedure is the introduction of 1 or 2 per cent of the sample solution into the vessel. Plug the vessel with a stopper and shake for some time before placing the Anderson pipette in the vessel and allow separating the 10 ml sample at different time intervals. The retained samples are evaporated and weighed for the constant weight. The particle size is calculated by using Stoke's law.
The advantages are that this method is relatively simple and inexpensive. This provides accurate and reproducible values. The limitations of this method are as follows:
- Large particles creates turbulence
- Temperature control is necessary
- Particle reaggregation is observed
- Less solubility of the compounds
Zone Sensing Method
This method is commonly known as the correct. In this method, the number and size of the particles which are suspended in the electrolyte solution is determined by passing through the orifice which is placed in the electrolyte solution. The electrodes are immersed in the electrolyte solution. The sample solution passing through the orifice generates a voltage difference which is measured by the pulse processor. The voltage pulse magnitude is directly proportional to the particle size. This method is used for the determination of particle sizes in the range of 30 μm to 2000 μm.
Zone sensing apparatus
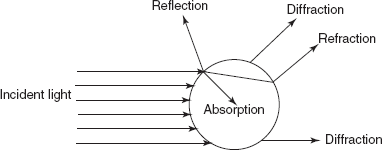
Light Scattering Method
This method is mainly based upon the principle of scattering. The particles present in the sample may absorb, reflect, refract or diffract the incident light.
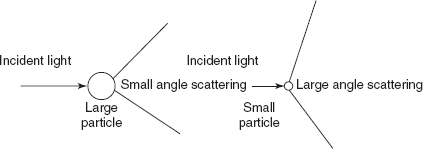
The large particles show a small angle of scattering and the small particles show a large angle of scattering.
The particles pass through the laser beam, and the light scattered by the particles are collected and detected by the detector. The particle scattering intensity is directly proportional to the particle size.
The advantages of this method are as follows:
- Excellent reproducibility
- Accurate
- Simple to use
- Highly versatile
The main disadvantage is that it is expensive.
Light scattering phenomenon
Light scattering angle difference
Scatter apparatus
APPLICATIONS
- Used in stability studies
- Used in the determination of excipient physical parameters
- Used in the determination of the processability
- Used in the determination of the flow properties of powders
- Used in the determination of product suitability
REVIEW QUESTIONS
- What are the different diameters used in particle size analysis?
- Explain the sedimentation method.
- Explain the factors that affect the method selection for particle size analysis.
- What is the principle involved in the microscopic method?
- Write about the principle involved in the light scattering method.
Comments