Free pharmacy material



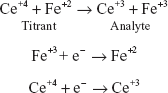
Redox Titration
INTRODUCTION
This titrimetric method is mainly based upon the change of the oxidation number or electrons transfer between the reactants, that is, these reactions are mainly based upon the oxidation-reduction reactions. In oxidation-reduction titration method, a reducing substance is titrated with standard solution of an oxidizing agent (e.g., ceric ammonium sulphate) or an oxidizing substance is titrated with the standard solution of the reducing agent (e.g., titanous chloride).
PRINCIPLE
The principle involved in the oxidation-reduction titrations is that the oxidation process involves the loss of electrons whereas the reduction process involves the gain of electrons.
Oxidant + ne ↔ Reductant
The redox titration is mainly based upon the oxidation of the analyte by the oxidizing agent and the oxidation and reduction of the reaction is determined by the indicators or by potentiometric.
The permanganate ion undergoes the following reaction:
MnO4− + 8H+ + 5e−  Mn+2 + 4H2O
Mn+2 + 4H2O
Purple mangnate ion ↔ Colourless manganese ion
The dichromate ion undergoes the following reaction:
Cr2O7−2 + 14H+ + 6e−  2Cr+3 + 4H2O−
2Cr+3 + 4H2O−
Orange dichromate ion ↔ Green chromium ion
At the end point,
ΔE = 0
THEORY
The oxidation leads to the increase in the oxidation number and reduction leads to the decrease in the oxidation number. Oxidation process involves the loss of electrons while the reduction process involves the gain of electrons, that is, oxidizing agents undergo reduction and reducing agents undergo oxidation.
Oxidant + ne−  Reductant
Reductant
Reductant  Oxidant + ne−
Oxidant + ne−
Ce+4 + Fe+2  Ce+3 + Fe+3
Ce+3 + Fe+3
Ce+4 + e−  Ce+3 (Reduction)
Ce+3 (Reduction)
Fe+2  Fe+3 + e− (Oxidation)
Fe+3 + e− (Oxidation)
The oxidation of the substance increases the oxidation number and the reduction of the substance decreases the oxidation number. The ability of the compound to accept or lose electrons is expressed by the standard electrode potential. This helps in the determination of ions which undergoes oxidation or reduction. Standard electrode potential is used in the following:
- Calculation of cell potentials:
- ECell = ERight − ELeft
- Example:
- Ag/Ag+//Cu+2/Cu
- ECell = ECu+2 − EAg+
- = 0.2867 − 0.6984
- = −0.0412 V
- Calculation of redox equilibrium constant:
- Cu+ + 2Ag+
Cu+2 + 2Ag
- Therefore,
The electrode is commonly employed for the determination of the oxidation and reduction changes in the reaction.
The electrode potential of the electrode when it is immersed in the mixture of oxidant-reductant solution is given by the following equation:
where ET = observed potential; E0 = standard potential; R = constant; T = temperature; n = number of electrons involved in the oxidation or reduction.
OXIDISING AND REDUCING AGENTS
- Oxidizing agents: The following are the commonly employed oxidizing agents in redox titrations:
- KMnO4 in dilute H2SO4:
- MnO4− + 8H+ + 5e−
Mn+2 + 4H2O
- K2Cr2O7 in dilute H2SO4:
- Cr2O7−2 + 14H+ + 6e−
2Cr+3 + 4H2O
- Iodine solution:
- I2 + 2e−
2I−
- Reducing agents: The following are the commonly employed reducing agents in the redox titrations:
- Mohr's salt (FeSO4−(NH4)2SO4−6H2O)
- Fe+2
Fe+3 + e−
- Oxalic acid (H2C2O4−2H2O)
- Cr2O7−2
2CO2 + 2e−
- Sodium thiosulphate (Na2S2O3−5H2O)
- 2S2O3−2
S4O6−2 + 2e−
Equivalent weights of the oxidizing and reducing agents are as follows:
The equivalent weight is given by the following formula:
The equivalent weight of the reducing agent is defined as the weight that loses electrons equivalent to 96500 C.
Fe+2  Fe+3 + e−
Fe+3 + e−
The equivalent weight of ferrous to ferric is 151.919.
The equivalent weight of the oxidizing agent is defined as the weight that gains electrons equivalent to 1 faraday
Ce+4 + e−  Ce+3
Ce+3
The equivalent weight of ceric sulphate is 332.24.
FACTORS AFFECTING REDOX TITRATIONS
The only factor that affects the redox titration is pH.
Example: KMnO4 acts as an oxidizing agent in the alkaline medium, neutral medium, and acidic medium. However, it acts as a strong oxidizing agent in the acidic medium.
The factors that affect the redox titration curves are as follows:
- Reactant concentration: The ability of the reactant undergoes oxidation or reduction.
- Completeness of the reaction: The incompleteness of the reaction shows the depression in the titration curve.
REDOX INDICATORS
Redox indicator should be able to possess the sudden change at the equivalence point during the redox titration. It should be capable of undergoing the oxidation and reduction.
InOxidation + ne  InReduction
InReduction
Some indicators are specific to the compounds. They react with one of the reactants in the titration to produce the colour.
Example:
|
Starch reacts with the iodine to produce deep blue colour.
|
Potassium thiocyanate (KSCN) reacts with iron to produce red colour.
|
There are different types of redox indicators. They are as follows:
- Based on the addition of the indicator:
- Self indicators: The titrant itself acts as a self indicator. It shows the intense colour at the end point.
- Examples: Potassium permanganate—end point is pink to colourless.
- Iodine—end point is brown to black colour
- Ceric ammonium sulphate—end point is colourless to yellow colour
- Internal indicators: These are added to the reaction mixture during the titration.
|
Phenanthroline blue
|
|
Methylene blue, etc.
|
External indicators: These are used externally by means of grooved tile and then mixed with the indicator solutions.
Example: Ferrous ions in dichromate solution show the Prussian blue colour with potassium ferricyanide solution.
Based on the nature of the indicator:
- Metal organic complexes:
- Example: Phenanthroline shows the colour change from blue to red.
- Free organic complexes:
- Example: Methylene blue shows the colour change from blue to colourless.
Based on the dependence on the pH:
- pH independent indicators:
|
2,2’-bipyridine shows the colour change from colourless to yellow.
|
|
5,6-dimethylphenanthroline shows the colour change from yellow-green to red.
|
pH dependent indicators:
|
Safrannin T shows the colour change from red-violet to colourless.
|
|
Neutral red shows the colour change from red to colourless.
|
Based on the instrument used for the endpoint detection: This method is mainly based upon the conductivity determinations and potential determinations at the end point. In this method, platinum electrode is used as the indicator electrode and the glass electrode is used as the reference electrode.
Example: conductometry determinations.
TYPES OF REDOX TITRATIONS
- Based on the titrant used:
- Permanganate titration: The reducing substances are determined directly by the potassium permanganate and the oxidizing substances are determined indirectly.
- Example: 2KMnO4 +10FeSO4 + 8H2SO4
5Fe2(SO4)3 + K2SO4 + 2MnSO4 +8H2O
- Dichromate titration: The solution which is prepared from the potassium dichromate is stable and acts as strong oxidizing agent.
- Example: 2K2Cr2O7 +6FeSO4 +7H2SO4
3Fe2(SO4)3 +K2SO4 +Cr2(SO4)3 +7H2O
- Iodine titration:
- Direct method: Iodine is used as the titrating agent.
- Indirect method: The liberated iodine is back titrated with the sodium thiosulphate.
- Based on the method:
- Direct titration: Some substances are initially coloured and the indicator solution is not necessary for the end point determination.
- Example: Azo dyes and quinones are titrated by this method.
- Back titration: An excess volume of the titrant solution is added to the sample solution and then the excess titrant is back titrated with the other titrant solution.
- Example: Chloramphenicol is titrated by this method.
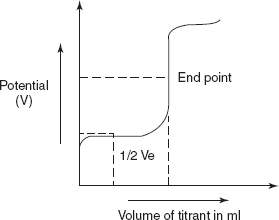
TITRATION CURVE
The titration curve in the redox titrations is mainly based upon the oxidation-reduction reaction between the analyte and the titrant.
Example:
The above reaction is determined by potentiometrically using platinum and calomel electrodes. Then the titration curve of the above reaction shows the three regions by titrating the iron ions with cerium ions.
Titration curve
The three regions are as follows:
- Before the end point the potential is created by the analyte.
- At the end point the potential at the indicator electrode is increased rapidly.
- After the end point the potential is determined by the titrant.
PROCEDURE FOLLOWED FOR REDOX TITRATION
Reaction with Potassium Permanganate Solution (Oxidizing Agent)
This is the powerful oxidizing agent which is introduced by F. Margueritte. The reaction mechanism is as follows:
MnO4− + H+ + 5e−  Mn+2 + 4H2O
Mn+2 + 4H2O
- Preparation of the permanganate solution: The accurate amount of the potassium permanganate is weighed on a watch glass and then it is transferred into the beaker. Then this is dissolved in the three-fourth portion of the distilled water and the mouth of the beaker is covered with the appropriate stopper. The resulting solution is boiled for 15-30 minutes and is allowed for cooling to room temperature. The solution is filtered through a cotton plug and is made to desired volume by adding distilled water.
- Standardization of the potassium permanganate solution: The standardization of the potassium permanganate solution is done by the arsenic oxide solution or by sodium oxalate solution.
- Applications of the potassium permanganate:
- Used in the analysis of metallic peroxides: To the 100 ml of distilled water concentrated sulphuric acid and boric acid is added and then the mixture is cooled in the ice bath and then the sample solution is added. The appropriate quantities are taken and titrated with the standard permanganate solution.
- 2MnO4− + 5NO2 + 6H+
2Mn+2 + 5O3− + 8H2O
- Used in the determination of the nitrites: Commercial potassium nitrite is weighed accurately and dissolved in the cold water and is made to the desired volume with the distilled water. Then the desired volume of the standard potassium permanganate solution is taken and then appropriate volume of the sulphuric acid is added. Then the nitrite solution is taken in the burette and the permanganate solution is titrated until it decolourizes.
- 2MnO4− + 5NO2 + 6H+
2Mn+2 + 5NO3− + 3H2O
Reaction with the Titanous Chloride Solution (Reducing Agent)
This is the strong reducing agent. The reaction is as follows:
Ti+3 + H2O  2H+ + e−
2H+ + e−
- Preparation of the standard titanous chloride solution: The appropriate volume of the titanous chloride solution is taken and then this solution is added to the equal volume of the hydrochloric acid and then it is made up to the appropriate volume with the cool distilled water.
- Standardization of the titanous chloride solution: The standardization of the titanous chloride is carried by using the ferric ammonium sulphate. The appropriate volume of the ferric ammonium sulphate is taken and the carbon dioxide stream is passed until the air has been removed. Then this solution is titrated with the standard titanous chloride solution.
Reaction with the Ceric Sulphate (Oxidizing Agent)
This is a strong oxidizing agent in the presence of acidic conditions. The titrations involving the ceric sulphate as oxidizing agent are named as ceriometric titrations.
Ce+4 + e−  Ce+3
Ce+3
Preparation of 0.1 M ceric sulphate solution: 35 g of ceric sulphate is weighed accurately and sulphuric acid and water is added to it. Then it is warmed gently to dissolve the salt. The appropriate volume is made up with the distilled water.
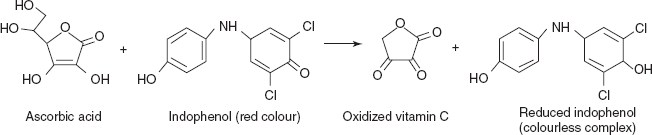
Titrations Involving 2,6-dichlorophenol Indophenols
This is mainly used in the titration of vitamin C with 2,6-dichlorophenol indophenols.
The advantages are as follows:
- Easy to handle
- Stability is high
- Color production is appropriate
The disadvantages are as follows:
- Requires skilled people to determine the end point
- Cost effective
APPLICATIONS
- Used in the determination of phenols.
- Method: There are different methods followed by the different persons but phenols are oxidants. So these should be titrated with the reducing agent.
- Used in the determination of iron in limonite.
- Method: Accurately weighed sample of iron is added to the 1 ml of HCl and the solution is heated gently. Then SnCl2 is added drop wise until yellow colour disappears. The resulting solution is added to the 5 ml of distilled water and 2 ml of the HgCl2. The formation of the white colour precipitate indicates the completion of the reaction. To this resulting solution 15 ml of water, 0.4 ml of sulphuric acid, and 1.2 ml of phosphoric acid solution and little quantity of redox indicator are added. Finally it is titrated with the potassium dichromate as the titrant.
- 6Fe+2 + 14H+ + Cr2O7−2
6Fe+3 + 2Cr+3 + 7H2O
- Used in the determination of calcium in lime stone.
- Method: Sample is dissolved in the strong acid like nitric acid and the resulting solution is titrated with the standard potassium permanganate solution. End point is the appearance of the pink colour.
- Used in the determination of oxidizing rate of the pyrolusite.
- Method: Pyrolusite is commonly known as the impure manganese oxide. In this method the sample is mixed with the sodium oxalate solution and is diluted with the distilled water. Then concentrated sulphuric acid is added followed by the boiling of the solution to evolve the carbon dioxide. The carbon dioxide free solution is then titrated with the standard potassium permanganate solution. End point is the appearance of pale pink colour.
- Used in the determination of chromium in chromate.
- Used in the quantitative determination of metals.
- Example: Ca, Mg, Zn, Co, Ni, etc.
- Used in the determination of dissolved oxygen.
- Used in the determination of oxidation state of elements.
- Used in the analysis of adrenaline.
- Method: The drug is extracted with carbon tetrachloride and then starch solution and iodine solution is added and then sodium thiosulphate is also added and finally it is titrated with sodium bicarbonate.
- Used in the analysis of analgesics and antipyretics.
- Method: Sample is dissolved in 6 N of HCl and heated to 60° C. Then it is titrated with 0.05 N of potassium bromated solution using methyl red as indicator.
- Used in the analysis of isoniazid.
- Method: Drug is dissolved in water and added to the mixture of HCl and KBr and then it is slowly titrated with 0.0167 M of potassium bromate solution using methyl red as indicator. End point is until the red colour of indicator disappears.
- 1 ml of 0.0167 M potassium bromate ≡ 0.003429 g of drug
- Used in the analysis of menadione.
- Method: Titrimetric methods of this drug are mainly based upon the oxidation reduction reactions. The B. P employs titration with titanous chloride. The U. S. P involves reduction with zinc and HCl and then the reduced form is titrated with ceric sulphate using O-phenanthroline as indicator
- Used in the analysis of tocopherol.
- Method: 50 ml of the saponifed sample is mixed with the 50 ml of alcoholic sulphuric acid and 20 ml of water. To this two drops of diphenyl amine solution is added and titrated with 0.01 N ceric sulphate solution.
- 1ml ceric sulphate ≡ 2.363 g of tocopherol
REVIEW QUESTIONS
- Define oxidation-reduction reactions and list out the different oxidizing and reducing agents.
- Explain about the theory involved in the redox titrations.
- What are the different types of redox indicators?
- Explain the Nernst equation.
- What is standard oxidation potential?
- Give the principle and procedure involving the potassium permanganate as titrant.
- What are self-indicators? Explain with examples.
- Add a note on strengths of the oxidizing-reducing agents.
- What are the factors affecting redox titrations?
Comments
Very nice explained about Redox Indicators and also easy to understand all information you have provided.
@lobachemie
References please😊
My mother knew nothing [about HIV]. She didn’t understand anything. Do you know why? She didn’t have [the chance] to go out of the house and communicate with society. However, my father does interact with the community. I know his friends are mature and dignified africa america. So he has a better understanding than her.My father came call me on a sadfull day sitting on my couch about a friend of his from africa who introduce him to Dr Itua herbal cure in africa in which he advise we should purchase his herbal medicine to cure my hiv so we did and Dr Itua prescribed I should drink the herbal medicine for two weeks to cure although we were so curious about the whole thing ,I finished the herbal medicine like he advised then he talked to me to visit my nearest clinic for check up I did and now I'm totally cured from Hiv my father was my rock and I and my family are now happy together also Dr Itua has be helpful in my community ever since he cure my Hiv so why I'm leaving my story on here today is to reach out someone out here to hope on God and never give up no matter the situation you that you are facing especially through this pandemic seasons which has really taught us all on how we should be helpful to each other and cherish one another.Dr Itua cures the following diseases..... Herpes,Liver cancer,Throat cancerLeukemia.,Alzheimer's disease,Chronic Diarrhea,Copd,Parkinson,Als,Adrenocortical carcinoma Infectious mononucleosis.
Intestinal cancer,Uterine cancer,Fibroid,Bladder cancer,Hiv,Esophageal cancer,Gallbladder cancer,Kidney cancer,Hpv,Lung cancer,Melanoma,Mesothelioma,Multiple myeloma,Oral cancer,Sinus cancer,Hepatitis A,B/C,Skin cancer,Soft tissue sarcoma,Spinal cancer,Stomach cancer,Vaginal cancer,Vulvar cancer,
Testicular cancer,Thyroid Cancer.You can contact Dr Itua Herbal Center on E-Mail: drituaherbalcenter@gmail.com Or Whats-App Chat : +2348149277967