Free pharmacy material




Voltammetry
INTRODUCTION
Voltammetry is the study of the current by applying the potential. This helps in the determination of the half-cell reactivity of the sample. The voltammetry was first proposed by the Jaroslav Herovsky in 1922 by the principle of polarography. The analytical advantage of the voltammetry is the sensitivity with different concentrations of the samples. The only interferences in the voltammetric measurements are dissolved oxygen and the solvent reduction. The main uses of the voltammetric measurements are the following:
- Used in the determination of the kinetic rates of the reactions.
- Used in the determination of the adsorption process on the surfaces.
- Used in the determination of the thermodynamic properties.
- Used in the determination of oxidation reduction process.
There are mainly two principle methods involved in the voltammetry. They are as follows:
- Polarography: Measurement of the current at different potentials.
- Amperometry: Measurement of the current at a fixed potential.
POLAROGRAPHY
Introduction
Polarography was first introduced by Heyrovsky in 1920. This is a voltammetric method. This method is the measurement of current (i) as a function of applied potential (E). This method is mainly used for the analysis of the electro-reducible or electro-oxidisable groups.
Principle
The main principle in the polarography is the reduction process taking place at the electrode. This method has limited sensitivity. The reduction at the electrode increases the voltage applied between the polarisable and non-polarisable electrodes and the current is recorded that is, the metallic ions are reduced at the surface of the electrode. Then the following three steps are observed:
- Migration of the ions from the solution to the electrode surface.
- Reduction of ions to form neutral atoms.
- Deposited atoms are converted to the crystal lattice.
Theory
The theory involved in polarography is when the working electrode is dipped in the analyte solution containing electro-active species, the following reduction takes place:
A(OX) + ne−  A(RED)
A(RED)
Example: Cu+2 + 2e−  Cu
Cu
The reduced potential is created on the working electrode. The movement of the ions from the solution to the electrode is by three mechanisms. They are as follows:
- Convection: This is also known as discharge process. This is carried out by the stirring of the sample solution at a constant temperature.
- Migration: Here movement of particles due to attraction of force of the electric field is created by the electrode.
- Diffusion: Here spontaneous movement of the sample ions occurs based on the concentration gradient.
- The movement of the sample ions is controlled by the placement of the supporting electrolyte solution.
|
Acids
|
|
Bases
| |
|
Buffers
| |
|
Salts
| |
|
Chelating agents
|
This supporting electrolyte solution surrounds the electrode with ions. The supporting electrolyte should posses the following ideal requirements:
- It should be chemically inert.
- It should have different discharge potentials.
- It should have ionic conductivity.
The total current flowing is given by the following equation:
I = Id + Im
where I is the total current; Id is the diffusion current; Im is the migration current.
The diffusion rate of the ion on the electrode surface is stated by Fick's second law:
δc/δt = Dδ2c/δx2
where D is the diffusion coefficient; C is the concentration; t is the time; x is the distance from the electrode surface.
Apparatus and the Methods
The apparatus consists of a dropping mercury electrode which acts as a cathode and as a working electrode. The anode used is the pool of mercury at the bottom of the reservoir which acts as a reference electrode. The reference electrode potential is constant. These two electrodes are placed in the sample solution which contains the both anions and cations. Then these anode and cathode are connected to the battery, voltammeter and galvanometer. Then apply the constant voltage and record the current–voltage curves using recorders. The sample cell is made of glass with tapering edge to place the mercury. The cathode capillary is dipped into the sample solution by setting the drop time of about 2–7 s. To control the movement of the ions to the surface on the electrode, the supporting electrolytes such as saturated potassium chloride solution are used. The oxygen present in the sample solution is removed by the alkaline pyrogallol solution. The determined diffusion current is directly proportional to the concentration of the sample solution.
Polarography apparatus
The current–voltage curves have the following advantages:
- Surface area is calculated by the weight of the drops.
- Reproducible values.
- Reduction potential is less.
Electrodes
The polarography is mainly composed of the three types of the electrodes. They are as follows:
- Working electrodes: The working electrode is mainly used for the determination of the analyte response to the potential.
- Example: Dropping mercury electrode
- Dropping mercury electrode: This electrode was first introduced by the Barker. The basic principle involved in this electrode is to control mercury flow through the capillary tube which is closed by the needle valve. The main advantage is that this electrode is applicable to +0.4 to −1.8 V. The main disadvantage of this electrode is the capillary blocking and poisonous mercury.
- Auxiliary electrode: It completes the circuit between the potentiostat and the working electrode.
|
Platinum electrode
|
|
Glass carbon electrode
|
Reference electrode: This electrode provides the reference potential for the working electrode and for the auxiliary electrode.
|
Silver–silver chloride electrode
|
|
Calomel electrode
|
Half-wave Potential
Half-wave potential is the important constant in the polarography. The half-wave potential is defined as the difference between the total current and the residual current which is equal to the one-half of the limiting current. This is denoted by E1/2. This is obtained from the current-voltage curve which shows the infection. This is mainly used in the identification of the substances.
The polarographic electrode reactions are divided into the following:
- Reversible reactions.
- Irreversible reactions.
The half-wave potential is equal to the oxidation–reduction potential.
The half-wave potential is determined by the following equation:
Eapp = E1/2 + (0.0592/n) log(id – i)/i
where Eapp is the applied potential; E1/2 is the half-wave potential; n is the number of electrons; id is the diffusion current; i is the current at the applied potential.
The following are the factors which affect the half-wave potential:
- Temperature of the analyte solution.
- Nature and concentration of the support electrolyte solution.
- Complex formation.
- Rate of electron transfer.
- Salt concentration.
Half-wave potential plot
Different Currents in the Polarography
- Residual current: The current that flows in the absence of the sample material is known as the residual current. This is due to the presence of impurities. This is denoted by ir.
- Migration current: This current is due to the migration of the oxidisable or reducible ions which is proportional to the potential gradient. This is because of the proportion of the analyte. This can be removed by the addition of the supporting electrolyte solution. This is denoted by im.
- Limiting current: This is total of the diffusion current and the migration current. This is caused by the depletion of the oxidisable or reducible ions at the electrode surface. In this, the current is steady where the diffusion of ions is equal to the rate of reduction. This is reached by the complete saturation of the electrode. This is denoted by ii.
- Diffusion current: This is obtained by the diffusion of the ions from the sample solution to the surface of the electrode. This is mainly due to the concentration gradient. This is denoted by id.
- The following are the factors affecting the diffusion current:
- Diffusion current is directly proportional to the concentration.
- Temperature is directly proportional to the temperature.
- Viscosity of the medium is inversely proportional to the diffusion current.
- The presence of impurities decreases the diffusion current.
Types of Polarography
There are several types of the polarographic methods but the important types are the following:
- Rapid direct current polarography: In this, the mercury drop rhythmatically falls from the electrode.
- Sampled direct current polarography: Initially the potential is increased and maintained constantly throughout the process.
- Pulse polarography: The potential pulse is imposed at the end of the process. These are of two types:
- Differential pulse polarography.
- Normal pulse polarography.
Methods of Quantitative Analysis
There are four methods for the quantitative analysis:
- Direct comparison method: In this method, the diffusion current of the unknown sample concentration is determined by comparing it with the reference which is done by a compound of known concentration. In this method, the conditions are maintained at optimum temperature.
- The diffusion current is given by the following equation:
- IdS/IdR = CS/CR
- where IdS and CS are the diffusion current and the concentration of the sample, respectively; IdRand CR are the diffusion current and the concentration of the reference, respectively.
- Multiple standard method: Same as the direct comparison method where the series of the standard solutions diffusion current and the unknown solution diffusion current are measured. Then plot the graph between the standard diffusion current values and concentration, which will produce the straight line. From this plot, the concentration of the unknown solution can be determined.
- Internal standard method: This method is also known as the pilot ion method. In this method the unknown ion concentration is measured by using the internal standard. This concentration is determined by the following equation:
- I dS/I dR = I a C S/Ib CR
- where Ia/Ib is known as the pilot ion ratio.
- Standard addition method: In this method initially the sample solution's diffusion current is measured and then the standard solution of the known quantity is added to the sample solution and again the diffusion current is measured. Then the concentration of the sample solution is measured from the following equation:
- where C is the concentration; i is the diffusion current; v is the volume of the sample and the standard solutions.
ADVANTAGES
- Minute sample is required for the analysis.
- Time consumption is less.
- Easy handling.
- High sensitivity.
DISADVANTAGES
- Less accurate.
- Skilled person is required.
APPLICATIONS
- Used in the determination of the composition of the alloys.
- Used in the qualitative determination of the elements.
- Used in the estimation of the trace metals like Zn, Fe, Mn and Cu.
- Used in the determination of the free sulfur in petroleum fractions.
- Used in the determination of the vitamin C in the food beverages.
- Used in the functional group analysis.
- Used in the determination of the complex compositions.
- Used in the determination of the dissolved oxygen in the gases.
- Used in the determination of the local anesthetics.
AMPEROMETRY
Introduction
The amperometric measurements are mainly based on the polarographic principles and the measurement of the current at a fixed potential. This is mainly resulting from the electrochemical oxidation or reduction of the sample which is electro-active. The diffusion current is directly proportional to the concentration of the electro-active substance in the solution.
Principle and Theory
The main principle involved in the amperometry is the measurement of the current between the working and the counter electrodes which is induced by the redox reaction. The electrochemical cell in the amperometry is mainly composed of three electrodes. They are as follows:
- Working electrodes: The working electrode is mainly used for the determination of the analyte response to the potential.
- Example: Dropping mercury electrode
- Dropping mercury electrode: This electrode was first introduced by the Barker. The basic principle involved in this electrode is the control of mercury flow through the capillary tube which is closed by the needle valve. The main advantage is that this is electrode is applicable to +0.4 to −1.8 V The main disadvantage of this electrode is the capillary blocking and poisonous mercury.
- Auxiliary electrode: It completes the circuit between the potentiostat and the working electrode.
|
Platinum electrode
|
|
Glass carbon electrode
|
Reference electrode: This provides the reference potential for the working electrode and the auxiliary electrode.
|
Silver–silver chloride electrode
|
|
Calomel electrode
|
The theory involved in this is when the working electrode is dipped in the analyte solution containing the electro-active species, the following reduction takes place:
A(OX) + ne− A(RED)
Example: Cu+2 + 2e−  Cu
Cu
The reduced potential is created on the working electrode. The potential of the working electrode is controlled with reference to the reference electrode. Then the current is flowed between the working electrodes and the auxiliary or control electrode. The use of the three electrodes avoids the back potential which is caused by the IR drop. The total current flowing is given by the following equation:
I = Id + Im
where I is the total current; Id is the diffusion current; Im is the migration current.
The diffusion rate of the ion on the electrode surface is stated by Fick's second law:
δ c/δ t = Dδ2c/δ x2
where D is the diffusion coefficient; C is the concentration; t is the time; x is the distance from the electrode surface.
Amperometric Titrations
In amperometric titration, the titration of the electro-reducible or non-reducible compound is determined by the titration of the titrant which contains the counter ions which are common in titrates. This raises the diffusion current. At the end point, there is a sharp change in the diffusion current based on the concentration of the electro-active substance. The titration curve is plotted between the diffusion current and volume of the titrant. The following are the conditions for the titration:
- Electro-reducible compounds should be present in the solution.
- The potential applied should be of limiting current.
Amperometric titration curve
Instrument for the Amperometric Titration
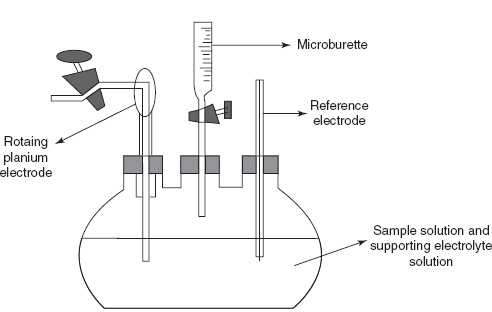
The apparatus consists of a dropping mercury electrode which acts as the cathode and as the working electrode. The anode used is the pool of mercury at the bottom of the reservoir which acts as the reference electrode. The reference electrode potential is constant. These two electrodes are placed in the sample solution which contains both the anions and the cations. Then these anode and cathode are connected to the battery, voltammeter and galvanometer. Then apply the constant voltage and record the current–voltage curves using recorders. The sample cell is made up of glass with tapering edge to place the mercury. The cathode capillary is dipped into the sample solution by setting the drop time of about 2–7 s. To control the movement of the ions to the surface of the electrode, the supporting electrolytes such as saturated potassium chloride solution are used. The oxygen present in the sample solution is removed by the alkaline pyrogallol solution. The determined diffusion current is directly proportional to the concentration of the sample solution.
Dropping mercury electrode
The procedure is the titration cell is flled with the known volume of the sample and then add required amount of the supporting electrolyte solution. Then the cell is connected to the electrodes. Then the sample solution is titrated with the reagent which is present in the microburette. The dissolved oxygen is removed by the flow of the nitrogen gas. Then the current flow and the volume of titrant consumed are noted. The end point is determined by the graph which is indicated by the intersection of the lines in the graph.
Amperometer
Factors Affecting the Amperometric Titrations
- Concentration is directly proportional to the diffusion current that is diffusion current increases with the increase in the concentration.
- Potential maintenance.
Types of Amperometric Titrations
There are mainly five types of amperometric titrations. They are as follows:
- Titration of electro-reducible ion with the non-reducible ion: In this method, the electro-reducible titrate is titrated with the non-reducible titrant. The diffusion current is observed by the electro-reducible ions. The first part of the plot shows the decrease because of the decrease in the electro-reducible ions which are by the precipitation of the reducible ions. At the end point, all the reducible are removed and show the constant diffusion current.
- Example: Lead ions are titrated with the sulphate ions.
- Titration curve 1
- Titration of non-reducible ion with the electro reducible ion: In this method, initially the diffusion current of the non-reducible ions are minimum. The addition of the titrant to the titrate does not show the change in the diffusion current. At the end point, the further addition of the titrant shows the gradual increase of the diffusion current.
- Example: Titration of the chloride ions with the silver ions.
- Titration curve 2
- Titration of electro reducible ion with the electro-reducible ions: The titration of the reducible ions with the reducible ions shows the decrease in the diffusion current. This is because of the decrease in the concentration of the titrate. This titrate is precipitated with the titrant. Then the addition of the titrant shows the increase in the diffusion current because of the increase in the concentration of the titrant which increases the diffusion current. The curve shows the V-shaped curve.
- Example: Titration of the lead ions with the dichromate ions.
- Titration curve 3
- Titration involving the redox reaction: This method is mainly based on the oxidation and reduction properties of the substances. In this method, initially the oxidant is taken as the titrate and it is titrated with the reductant as titrant. The plot shows the decrease in the diffusion current due to the decrease in the concentration of the oxidant. When the diffusion current reaches minimum, the reductant added to the oxidant is completely oxidised by the oxidant. The end pint shows the intersection of the two lines.
- Example: Titration of the ferric ions with the titanous ions.
- Titration curve 4
- Titration with two indicator electrodes (Biamperometry): This method is mainly used when the redox system is present before and after the end point. In this method, the two electrodes are immersed in the electro chemical cell. Then the constant potential is applied among these electrodes. At the end point, one electrode is depolarised until the oxidant or reductant is completely oxidised or reduced. This method was first introduced by the Foulk and Bawden in 1926. The main advantage of this method is simple when compared to other methods.
- Example: Water titration with Karl Fischer reagent.
- Biamperometric titration curve
ADVANTAGES
- End point detection is easy.
- Very dilute solutions can be readily determined.
- Automation is easy.
- Inexpensive apparatus.
- Temperature independent.
- High selectivity.
DISADVANTAGES
- Inaccurate results are obtained some times because of interferences.
- Requires specific equipment.
- It should have voltammetric information.
APPLICATIONS
- Used in the water analysis by Karl Fischer reagent.
- Used in the quantitative analysis of the mixture of metal ions.
- Used in the analysis of alloys.
REVIEW QUESTIONS
- What is the principle involved in the polarography?
- Explain the theory involved in the polarography.
- What are the requirements for the electrolytic solution?
- What is total current and how it can be calculated?
- Explain about Fick's second law.
- Write about the connection of the apparatus used for the polarographic measurements.
- Explain the principle of the dropping mercury electrode.
- What is half-wave potential?
- What are the factors affecting the half-wave potential?
- What are the different types of currents?
- What are the types of polarography?
- What are the different methods used for the polarographic measurements?
- Explain the applications of polarography.
- What is the principle involved in the amperometry?
- Explain the theory of amperometry.
- Explain the instrumental components used in the amperometry.
- What are the factors affecting the amperometric titrations?
- What are the different types of amperometric titrations?
- What are the advantages and disadvantages of amperometry?
- List out the applications of amperometry.
Comments