Free pharmacy material






Quinolone Antibacterials
.1 INTRODUCTION
Quinolones constitute a large class of synthetic antimicrobial agents that are highly effective in the treatment of many types of infectious diseases, particularly those caused by bacteria. Quinolones are potent, broad-spectrum antibacterial agents. The early congeners (non-fluorinated at C-6 like nalidixic acid) were limited to certain gram-negative infections such as urinary tract infections. However, the modern generation of fluoroquinolones containing C-6 fluoro substituent and a cyclic basic amine moiety at C-7 surpass their predecessors in terms of spectrum of activity and potency. This has allowed for their use against a variety of gram-negative as well as some gram-positive pathogens. Quinolones are relatively easily prepared and easily administered via parenteral and oral routes, and are well tolerated.
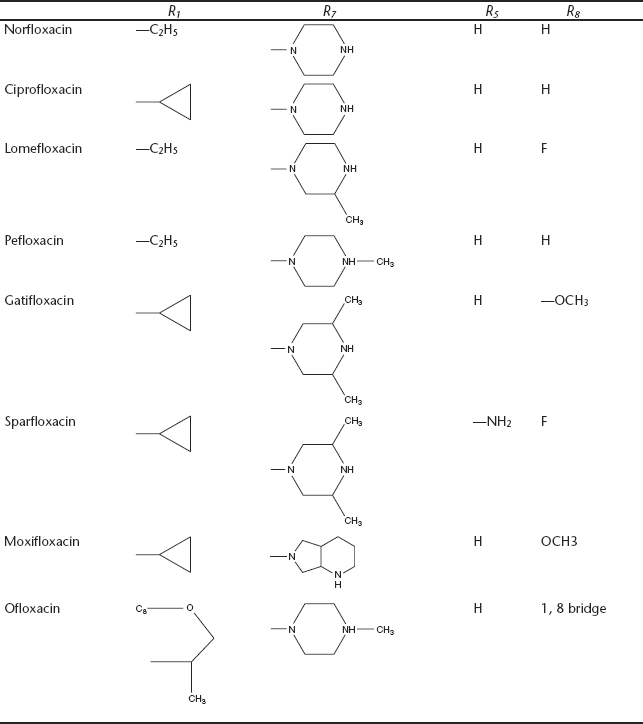
25.2 STRUCTURES OF FLUOROQUINOLONES
25.3 MECHANISM OF ACTION
Quinolones inhibit the action of bacterial DNA gyrase enzyme. This enzyme is responsible to supercoil and compact bacterial DNA molecules into the bacterial cell during replication. This action is accomplished by modifying the topology of DNA via supercoiling and twisting of these macromolecules to permit DNA replication or transcription.
.4 USES
Fluoroquinolones are used to treat upper and lower respiratory infections, gonorrhoea, bacterial gastroenteritis, skin and soft tissue infections, urinary tract infections, and bone and joint infections, and against tuberculosis.
.5 CLASSIFICATION
1st generation: Cinoxacin, flumequine, nalidixic acid, oxolinic acid, piromidic acid, pipemidic acid
2nd generation: Ciprofloxacin, enoxacin, lomefloxacin, nadifloxacin, norfloxacin, ofloxacin, pefloxacin, rufloxacin
3rd generation: Balofloxacin, grepafloxacin, levofloxacin, pazufloxacin, sparfloxacin, temafloxacin, tosufloxacin
4th generation: Clinafloxacin, gemifloxacin, moxifloxacin, gatifloxacin, sitafloxacin, trovafloxacin
In development: Ecinofloxacin, prulifloxacin
Veterinary use: Danofloxacin, difloxacin, enrofloxacin, marbofloxacin, orbifloxacin, sarafloxacin
25.6 SAR OF QUINOLONES
- Substituent at N-1 position: A compilation of active N-1 quinolone substituents is shown below with an emphasis on overall in vitro potency.
- The simple replacement of C-2 hydrogen has generally to be disadvantageous - e.g., C-2 methyl or hydroxyl groups. However, some derivatives containing a suitable C-1, C-2 ring have been shown to possess notable activity.
- Without doubt, the C-3 carboxylic acid moiety is most commonly encountered. Other acidic groups such as sulphonic acid, phosphonic acid, tetrazole, as well as derivatization as an ester results in a loss of antibacterial activity.
- The C-4-oxo group of the quinolone nucleus appears to be essential for antibacterial activity. Replacement with 4-thioxo or sulphonyl group leads to a loss of activity.
- The incorporation of an amino group at the C-5 position has proven beneficial in terms of antibacterial activity. The order of activity at R5: NH2, CH3 > F, H > OH, OR, SH, SR.
- The incorporation of a fluorine atom at the C-6 position of the quinolone is monumental. The order of activity at R6: F > Cl, Br, CH3 > CN.
- The introduction of a piperazine moiety at C-7 was a landmark development. Other aminopyrrolidines also are compatible for activity.
- A hydrogen atom at the C-8 or a nitrogen atom (a naphthyridone) is the most common. In general, a C-8 fluoro substituent offers good potency against gram-negative pathogens, while a C-8 methoxy moiety is active against gram-positive bacteria. The order of activity at R8: F, Cl, OCH3 > H, CF3 > methyl, vinyl, propargyl.
- A halogen (F or Cl) at the 8- position improves oral absorption.
- The joining of N1-group to the C-8 position with oxazine ring leads to active ofloxacin.
25.7 ADVERSE EFFECTS
Some side-effects of the quinolones are class effects, and cannot be modulated by molecular variation. Most of the fluoroquinolones produce photosensitivity reactions and cause convulsions, particularly concurrent administration of NSAID fenbufen. This effect is strongly influenced by the C7 substituent, with simple pyrrolidines and piperazines the worst actors. Increasing steric bulk through alkylation ameliorates these effects. Phototoxicity is determined by the nature of the 8-position substituent with halogen causing the greatest photo reaction, while hydrogen and methoxy show little light induced toxicity. Arthralgia and joint swelling have developed in children receiving fluoroquinolones; therefore, these drugs are not generally recommended for use in prepubertal children or pregnant women. Genetic toxicity is controlled in additive fashion by the choice of groups at the 1, 7, and 8 positions.
25.8 SYNTHESIS OF FLUOROQUINOLONES
β-Keto ester is formed by the reaction between (sub) benzoyl chloride and magnesium salt of diethyl malonate. This keto ester on reaction with triethyl ortho formate gives methoxy methylene derivative, which in turn reacts with corresponding amine (ethylamine/cyclopropylamine) to give aminomethylene derivative. Base cyclization of this compound gives quinolone-3-carboxylic acid derivatives. Reaction with appropriate piperazine affords titled compound.
Norfloxacin, ciprofloxacin, lomefloxacin, gatifloxacin, and sparfloxacin are prepared by this method by using appropriate starting material, primary amine and piperazine derivatives.
Ofloxacin (+/-)-9-Fluoro-2, 3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido [1,2,3-de]-1, 4-benzoxazine-6-carboxylic acid
Tetrafluorobenzoic acid on reaction with 1,1’-carbonyldiimidazole in tetrahydrofuran afforded the corresponding imidazolide, which, in situ, was treated with neutral magnesium salt of ethyl potassium malonate in the presence of tri-ethyl amine to yield ethyl 3-(2,3,4,5-tetrafluorophenyl)-3-oxopropanoate. Ethyl 2,3,4,5-tetrafluoro-α-[[(2-hydroxy-1-methyl-ethyl)amino]methylene]-β-oxo-benzenepropanoic acid (A) was prepared by a two-step one-pot reaction. First treatment of the keto ester with tri-ethyl orthoformate in acetic anhydride gave the one-carbon homologue enol ether intermediate ethyl α-(ethoxymethylene)-2,3,4,5-tetrafluoro-b-oxo-benzenepropanoic acid as an oil, which on reaction with (S)-(+)-2-amino-1-propanol at 0°C affords (A) as an oily residue. Compound (A) on cyclization with the base potassium carbonate/sodium hydride in dimethylsulphoxide yields ethyl 6,7,8-trifluoro-1,4-dihydro-1-(2-hydroxy-1-methylethyl)-4-oxo-3-quinolinecarboxylic acid. Further cyclization and hydrolysis of this compound is done by heating with 10 per cent aqueous sodium hydroxide in tetrahydrofuran, affording 9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid. This on further reaction with N-methyl piperazine in dimethylsulphoxide affords ofloxacin.
Levofloxacin It is the S- enantiomer (L-isomer) of ofloxacin, and has approximately twice the potency of ofloxacin, because the R + enantiomer (D-isomer) of ofloxacin is essentially inactive. In addition, the S- enantiomer (L-isomer) of ofloxacin has substantially less toxicity.
Nalidixic acid 1-Ethyl-7-methyl-4-oxo-[1,8]naphthyridine-3-carboxylic acid
Condensation of ethoxymethylenemalonate with 2-amino-6-methyl pyridine proceeds directly to naphthyridine; the first step in this transformation probably involves an addition-elimination reaction to afford the β-hydroxy ester. N-Ethylation with ethyl iodide and base hydrolysis affords nalidixic acid.
It is especially used in treating urinary tract infections, for example, by Escherichia coli, Proteus, Shigella, Enterobacter, and Klebsiella. It selectively and reversibly blocks DNA replication in susceptible bacteria.
Nalidixic acid is considered to be the predecessor of all members of the quinolone family, including the second, third, and fourth generations, commonly known as fluoroquinolones.