Free pharmacy material

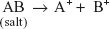
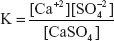
Precipitation Titrations
INTRODUCTION
Precipitation titrations are mainly based on the formation of the precipitate by the reaction of the sample with precipitating agents. The precipitate formed is the less soluble compound.
Ag+ + Cl−  AgCl (ppt.)
AgCl (ppt.)
The number of precipitating agents that can be used is limited because of the slow action to form the precipitate. This method is mainly used in the determination of the halides.
THEORY
The theory involved in the precipitation titration is the addition of the sample solution to the titrant in which the precipitating agent forms the precipitate and the end point is detected by the indicators.
Ag+ + I−  AgI (precipitate)
AgI (precipitate)
KI + AgNO3  AgI + KNO3 ↓
AgI + KNO3 ↓
The precipitating reagents are limited because of their less precipitate formation.
M + D ↔ MD
The precipitate formation constant is given by the following equation:
where {M} is the concentration of the sample ion, {D} is the concentration of the precipitating agent and {MD} is the concentration of the complex.
Solubility Product
Solubility product is defined as the product of the concentration of the ions increased to the appropriate range in a saturated solution at a constant temperature. This is mainly expressed for the sparingly soluble salt formed by the acid-base reaction. Consider the following reaction:
Then solubility product of the salt at constant temperature is given by the following equation:
Ksp = [A+][B+]
This is independent of the salt concentration.
Example: CaSO4  Ca+2 + SO4−2
Ca+2 + SO4−2
Then the equilibrium constant is written as the following:
Then it is simplified to get the solubility product of the salt at the saturation point at constant temperature.
Ksp = [Ca+2][SO4−2]
That is ion concentration is raised powers equal to the molar ratio of the compound.
Requirements for the Precipitation Indicators
- The colour change should occur rapidly.
- The colour change should take place with change in the titration curve.
Examples of indicators
- Chromate ion (Mohr's method)
- Fluorescein (Fajans method)
- Iron ion (volhard method)
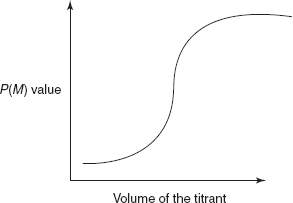
The plot between P(M) and the volume of the titrant shows the titration curve as the following.
Precipitation titration curve
The main three regions of the titration curve are as follows:
- Prior to the equivalence point.
- The equivalence point where the known amount of the titrant is added.
- The excess point of the titrant.
Conditions Required for the Precipitation Titrations
- It should be a very rapid reaction.
- It should be a single suitable stoichiometry reaction.
- It should show the change in the concentration of the reactants or products at the end point.
TYPES OF THE PRECIPITATION TITRATIONS
Based on the indicator used in the determination of the end point the precipitation titrations are classified into three types. They are as follows:
- Mohr's method: In this method potassium chromate solution is used as the indicator. This method is used in the determination of the Cl− or Br− by using silver nitrate as the titrant in neutral or slightly acidic condition. The end point is detected by the formation of the red silver chromate ion. This method is also known as the direct method.
- Ag+ + Cl−
AgCl (ppt.)
- Method: Sample is dried at 110 °C for 1 h and cooled in the desiccator. Then it is accurately weighed and is dissolved in the 100 ml of water. Then small quantity of the NaHCO3 is added to cease the effervescence. Then 2 ml of K2CrO4 is added as indicator solution and the resulting solution is titrated with the silver nitrate as the titrant. The end point is appearance of the red colour.
- 2Ag+ + CrO4−2
Ag2CrO4
- Volhard's method: In this method ferric ion is used as the indicator. This method is also known as the indirect method. The excess of the silver ions are titrated with the standard SCN− solution until red colour solution is appeared.
- Fe+ + SCN− ↔ Fe(SCN)+2
- This method's stability is increased by the two steps:
- Addition of nitrobenzene which shields the precipitate from aqueous medium.
- Filtration of the precipitate.
- Method: The sample solution and the solution of 1 ml saturated ferric ammonium sulphate as indicator are taken in the conical flask. Then the resulting solution is titrated with the 0.1 M of potassium thiocyanate solution until the dark red colour is appeared.
- Fajan's method: Fluorescein is used as the indicator. The mechanism involved is that the indicator is adsorbed to the surface of the precipitate and shows the colour change as reddish.
- Method: Dry the sample and NaCl in oven at 110 °C for 1 h and cool in the desiccator. Weigh 100 mg of the sample and NaCl in separate flasks and dissolve in the 100 ml of deionised water. Then 5 ml of 2% dextrin solution is added which stabilises the precipitate. Then 8-10 drops of the 0.1% of dichloroflourescein solution is added as the indicator to each flask. The resulting solution is then titrated with the silver nitrate solution as the titrant. The end point is the appearance of dark pink reddish.
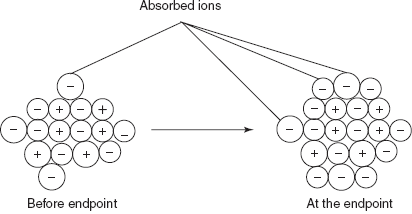
The other method for the end point detection is the Gaylussac method. In this method, the end point is determined by the formation of flocculation. The main cause for the flocculation is that the precipitate particles adsorb the common ions by creating a charge. At the end point, the formation of flocculation is observed.
End point detection
FACTORS AFFECTING THE PRECIPITATION TITRATIONS
- Nature of the solvent: Polarity is directly proportional to the precipitate formation. If the solvent is more polar then it enhances the precipitation, for example, water enhances the formation of precipitate.
- pH of the solution: The pH of the solution is directly proportional to the precipitation titrations. The more acidic solutions enhance the precipitate formation when compared to basic solutions.
- Concentration of the reactants: The concentration of the reactants is directly proportional to the precipitation reactions. Concentrated solutions form readily soluble precipitates where as the dilute solutions produce the unstable precipitates.
- Low solubility product formation during the titration: The formation of the low solubility product during the precipitation decreases the end point in the precipitation titrations. Hence this can be overcome by the adjustment of the pH.
- The nature and concentration of the foreign substances: The concentration of the foreign substances in the precipitation products decreases the stability of the product. Then the nature of foreign substances has the ability to form coprecipitation is taken into consideration. This situation can be overcome by the maintaining the optimum temperature throughout the titration.
- Temperature is directly proportional to the precipitation formation: If the temperature increases, the ability to form a precipitate also increases.
- Order of addition of the reagents: This plays an important role in the precipitate formation. Because some compounds require the pretreatment of the sample with the alkali or acid before addition of the precipitating agent and compounds requires the treatment with the alkali or acid after the addition of precipitating reagent.
- Example: In procaine analysis, the sample is first treated with the precipitating reagent such as tetra phenyl boron followed by the alkalising the solution with the standard NaOH solution.
ADVANTAGES
- Simple when compared to the other methods. The end point detection is also very simple when compared to other methods by the formation of the precipitate.
- Selectivity: This method is selective when the compounds are not able to determine by other methods.
- Specificity: These titrations are specific for specific compounds.
- Example: Determination of chlorides which gives the account of purity of the water.
- Less time consuming: These methods are less time consuming when compared to other methods which are done by directly adding the precipitating reagent to the sample solution and require small quantity of the titrant for the precipitate formation.
DISADVANTAGES
- It is used only for the quantitative determinations not for qualitative determinations.
- Interferences are more such as coprecipitation, occlusion, etc.
APPLICATIONS
- Used in the determination of the chloride ions.
- Method: Sample solution is mixed with the distilled water and the little quantity of the indicator solution such as potassium dichromate solution. Then titrate the solution with the standard silver nitrate solution and calculate the concentration of chloride ions in the sample as ppm AgCl precipitate.
- Used in the determination of the sulphate ions.
- Method: Sample is mixed with the distilled water and methanol. To this, add little quantity of the indicator solution. Then HCl is added until the solution turns to yellow colour. The resulting solution is titrated with the standard BaCl2 solution. The end point is the formation of the pink colour precipitate.
- Used in the determination of the epoxide.
- Used in the determination of the fatty acids.
- Used in the determination of the Zn.
- Method: Initially the zinc compound is precipitated with the precipitating agent. Then the precipitate formed is dissolved in the mineral acid and is titrated with the excess of silver nitrate solution. Then the excess of silver nitrate is titrated with the ammonium thiocyanate using ferric ammonium sulphate as indicator.
- ZnHg(CNS)4 + AgNO3
Zn(NO3)2 + Hg(CNS)2 + 2AgCNS + AgNO3 (Excess)
- Used in the determination of the fluoride ions.
- Used in the determination of the halides.
- Used in the determination of the organic substances in the food components.
REVIEW QUESTIONS
- What is the principle involved in the precipitation titrimetry?
- Explain the theory involved in the precipitation titrimetry.
- What are the different types of precipitation titrations?
- Explain Fajan's method with example.
- What are the factors affecting the precipitation titrations?
- Explain the different regions in the precipitation titrimetry.
- What are the applications of precipitation titrimetry?
Comments