Free pharmacy material








Spectro Fluorimetry and X-ray Fluorescence Spectroscopy
INTRODUCTION
Spectrofluorimetry is defined as the measurement and interpretation of the emission of the radiation after absorption. This emission of the radiation is generally called as the photoluminescence. This photoluminescence is divided into two types based on the time taken for the emission of the radiation.
- Fluorescence: The sample absorbs the radiant energy and emits the radiation immediately after the absorption of the radiation. The substances which show this phenomenon are called as fluorescent substances (light emitted within 10–12–10–9 s).
- Phosphorescence: The sample absorbs the radiation and emits the radiation continuously after absorption of the radiation. The time delay of the emission of the radiation is within 10–8 s.
PRINCIPLE
The main principle involved in the spectrofluorimetry and phosphorimetry is when an incident light absorbed by the sample, it undergoes the transition from the ground state to singlet excited state. Where the singlet excited state is not a stable one and the molecule present in this excited state immediately returns to the ground state by emitting the energy. This is the main principle of fluorescence.
In the case of phosphorescence, from the singlet excited state the molecule loses some part of energy and undergoes to a metastable triplet state and then returns to the ground state by emitting the energy known as phosphorescence.
THEORY
The electron which is present in the singlet ground state absorbs the radiation within the UV–visible region and undergoes transition and excited to the singlet excited state. This singlet excited state is relatively unstable and there are two possibilities, they are as follows:
- The excited molecules return immediately to the ground state by emitting the radiation (fluorescence).
- The excited molecules which are in singlet excited state again undergo the transition to a meta-stable triplet state and then return to the ground state by emitting the radiation.
Fluorescence and phosphorescence schematic diagram
The flourometric process involves the following steps:
- Excitation of the molecules by the UV radiation.
- Vibrational relaxation: The molecule present in the singlet excited state quickly loses the energy by the collision with the other molecules.
- Internal conversion: When the upper and lower electronic states have the same multiplicity, then it is called as internal conversion.
- Photon emission: The molecule in the singlet excited state returns to the ground state by the emission of the photon which is called as fluorescence.
- Energy transfer: The molecule returns to the ground state by the energy transfer.
- The total fluorescence intensity F is given by the following equation:
F = Iaφf
where Ia is the intensity of the light absorption; φf is the quantum efficiency of fluorescence which is defined as the part of the incident radiation emitted as fluorescence. The quantum efficiency is less than unity and it is a property of a molecule structure.
Since the I0 = Ia + It
where I0 is the intensity of the incident light; It is the intensity of the transmitted light.
Then Ia = I0–It
By substituting this value
F = (I0 − It)φf
From Beer–Lambert's law
It = I0e−εcl
F = I0(1 − e−εcl)φf
For weak fluorescent substances, the equation becomes
F = I0 × 2.3εclφf
TYPES OF FLUORESCENCE
The fluorescence is classified based on the emitted radiation wave length and based on the phenomenon. They are as follows:
- Based upon the emitted radiation: There are mainly three types. They are as follows:
- Stoke's fluorescence: The emitted radiation wave length is longer than the absorbed radiation wave length.
- Example: Conventional fluorimetry.
- Anti-Stoke's fluorescence: The emitted radiation wave length is shorter than the absorbed radiation.
- Example: Thermal fluorimetry.
- Resonance fluorescence: The emitted radiation wave length is equal to the absorbed radiation.
- Example: Mercury vapour at 254 nm.
- Based upon the phenomenon: There are three types. They are as follows:
- Sensitised fluorescence: When the elements such as thallium, zinc, cadmium are added to the mercury vapour, it is sensitised and produces the fluorescence.
- Direct line fluorescence: After the emission of the radiation, the molecules remain in the metastable state and finally come to the ground state.
- Step-wise fluorescence: The part of energy is lost by vibrational transition before the emission of the fluorescent radiation.
INSTRUMENTATION
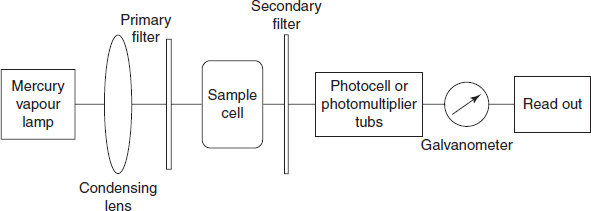
The basic components of fluorimeter are as follows:
- Radiation source: Generally the UV radiation sources are used for the excitation. Mostly the mercury vapour lamp is used.
- Filters: In fluorimetry, two types of filters are generally used to convert the polychromatic light to monochromatic light. The primary filter allows the UV radiation and absorbs the visible radiation for excitation, whereas the secondary filter allows the fluorescent radiation and absorbs the UV radiation.
- Sample cells: Generally quartz cells are used as sample cells.
- Detectors: The fluorescent radiation transmitted is measured by the detectors. Generally photovoltaic cells or photo multiplier tubes are used as detectors.
- Amplifier: A sensitive galvanometer is used as amplifier to measure the output of detector.
Spectrofluorimeter schematic diagra
Single-beam fluorimeter: Single beam fluorimeter consists of a mercury vapour lamp as radiation source and a condensing lens to pass the polychromatic light through the primary filter to convert the polychromatic light into monochromatic light. This monochromatic light of desired wavelength of radiation is passed through the sample container and is passed through the secondary filter. This secondary filter transmits the fluorescent radiation for measurement. This radiation is measured by the photomultiplier tube and is recorded by using the galvanometer.
Schematic diagram for the single-beam fluorimeter
Double-beam fluorimeter: In this, two beams are passed through two primary filters and simultaneously passed through the sample and reference. Then the fluorescent radiation is transmitted through the pair of secondary filters and then to the photomultiplier tube detector to record the ratio of two signals obtained from the sample and reference.
Schematic diagram for the double-beam fluorimeter
Spectrofluorimeter: It is generally known as Aminco–Bowman spectrofluorimeter. It consists of a high pressure xenon arc lamp as a radiation source which is passed through the excitation monochromator and irradiates with the sample and passes through the emission monochromator. Then the radiation emitted is detected by the photomultiplier detector. The signals obtained are recorded by using the recorder.
Double-beam spectrofluorimeter
FACTORS AFFECTING THE FLUORESCENCE INTENSITY
- Temperature and viscosity: The lower temperature and greater viscosity decrease the efficiency of the relaxation processes.
- Solvent and the pH: The use of polar solvents increases the fluorescence intensity. The pH increase shows the increase in the fluorescence intensity.
- Effect of the other solutes: The solutes containing the halogens or heavy atoms decrease the fluorescence.
- Substituent effect on fluorescence:
- The OH, NH2, NHR, NRR groups show the increased fluorescence.
- The COOH, CHO, N=N, I, Br, Cl and C≡N reduce the fluorescence intensity.
- Structure: Rigid structures exhibit more fluorescence than the normal structures.
- Example: Shows high fluorescence than the other compounds.
- Nature of the molecule: Rhe molecules with unsaturation show the high rate of fluorescence.
- Oxygen: The presence of oxygen molecule or oxidation process decreases the fluorescence intensity.
CONCEPT OF QUENCHING
Decrease in the fluorescence intensity is known as quenching. This is caused by the concentration, pH, chemical substances, temperature, viscosity, etc.
There are four types of quenching:
- Self-quenching: It is a phenomenon observed when solutions of high concentrations do not show a proportional increase in fluorescence intensity as shown at low concentrations. It is also called as concentration quenching.
- Collisional quenching: It is observed due to the increase in the number of collisions.
- Example: Presence of halides, heavy metals, increase in the temperature, and decrease in the viscosity.
- Static quenching: This type of quenching is observed as a result of complex formation.
- Example: The riboflavin fluorescence intensity is decreased by the complex formation with the caffeine.
- Chemical quenching: This type of quenching is due to various factors such as the change in pH, presence of oxygen, halides or heavy metals.
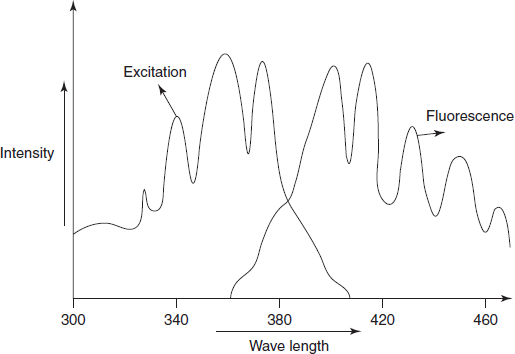
FLUORESCENCE SPECTRUM
The fluorescence spectrum contains the both excitation and emission spectra. The excitation spectrum contains the excitation radiation which excites the molecules from the ground state to the excited states. Thus the excitation spectrum is recording of the fluorescence versus the wavelength of the exciting or incident radiation. The emission spectrum is a measure of the relative intensity of the radiation emitted for returning of the excited molecules to the ground state. These absorption and emission spectra have the approximate mirror image relationship when the spacings between vibrational levels are equal or transition probabilities are equal.
Schematic diagram for the absorption and emission spectrum
The spectra for the fluorescence spectrum
ADVANTAGES
- Highly sensitive.
- High precision.
- Highly specific to the compounds.
- Most of the compounds are non-fluorescent compounds which are converted to fluorescent compounds by the use of fluorescent indicators.
|
Eosin green fluorescence
|
|
Fluorescein green fluorescence
| |
|
Quinine sulphate blue to violet fluorescence
| |
|
Acridine green to violet blue fluorescence
| |
|
2-Naphtha quinine blue fluorescence
|
LIMITATIONS
- Interferences are more.
- Examples: pH, oxygen, halides and heavy metals.
- Some times, the excitation source causes the chemical changes.
- All compounds are not fluorescent.
APPLICATIONS
- Used for the determination of the uranium salts in the field of nuclear research.
- Used in the determination of the inorganic ions.
|
Ruthenium in platinum metals
|
|
Aluminium in alloys
| |
|
Estimation of traces of boron in steel
|
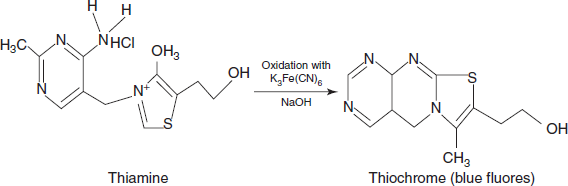
Used in the determination of the thiamine.
Reaction for the thiamine
Used in the determination of riboflavin.
Used in the study of the chemical equilibrium and kinetics.
Used in the determination of the diphenylhydantoin.
Reaction for the diphenylhydantoin
Used in the determination of the quinine in the urine samples.
Used in the determination of the morphine and codeine in the admixture.
REVIEW QUESTIONS
- What is the principle involved in the fluorescence?
- What are the fluorescent indicators?
- What are the different types of fluorimetry?
- What are the different factors affecting the fluorescence intensity?
- What is quenching? What are the different types of quenching?
- What is the theory involved in the measurement of the fluorescent intensity?
- What are the different applications of fluorimetry?
- What are the different transition states observed in the fluorimetry?
- List out the advantages and disadvantages of fluorimetry.
- What are the different components present in the spectrofluorimeter?
- Explain the process involved in the fluorimetric process.
X-RAY FLUORESCENCE SPECTROSCOPY
INTRODUCTION
This is non-destructive chemical analysis. This is mainly based on the interaction between the electron beams and X-rays with the samples. This is mainly used in the analysis of minerals. It is used in the surface analysis technique. In this method, X-rays are produced from the sealed tube to produce the secondary fluorescence by the sample, which gives the information about the nature of the atoms.
THEORY
The samples present in the ground state are excited with high-energy radiation with shorter wavelength such as X-ray radiation. This leads to the ionisation of the sample and goes to the higher energy state. The ions present in the higher energy state are not a stable one which returns to the lower energy state by emitting the radiation. This emitted radiation is known as the fluorescent radiation.
The basis for X-ray fluorescence is the different electron shells with the different number of electrons. Based on the number of electrons present in the shells they are classified as as follows:
Table for the types of shells present in the molecule
Number of electrons
|
Name of the shell
|
2 electrons
|
K-shell
|
8 electrons
|
L-shell
|
18 electrons
|
M-shell
|
32 electrons
|
N-shell
|
The K-shell takes more energy to lose the electrons. The electrons falling from the N-shell to K-shell emits more energy.
The following two steps are involved in the X-ray fluorescence spectroscopy:
Step I: The X-ray radiation hits the sample in the first step and loses one electron from the K-shell.
Step II: The electron from the L-shell replaces the lost electron in the K-shell. Then the energy is emitted by the replacement of the electron. The vacant K-shell is filled with the M-shell electrons.
INSTRUMENTATION
Flow chart of the X-ray fluorimeter instrument
The basic components of X-ray fluorimeter instrument are as follows:
- Radiation source: The X-ray tube is made up of Ag or Rh. The high energy electrons are produced at the anode.
- Filters: In fluorimetry, two types of filters are generally used to convert the polychromatic light to monochromatic light. The primary filter allows the UV radiation and absorbs the visible radiation for excitation, whereas the secondary filter allows the fluorescent radiation and absorbs the UV radiation.
- Sample cells: Generally quartz cells are used as sample cells.
- Detectors: The transmitted fluorescent radiation is measured by the detectors. Generally photovoltaic cells or photomultiplier tubes are used as detectors.
- Amplifier: A sensitive galvanometer is used as an amplifier to measure the output of detector.
ADVANTAGES
- Used for the single time analysis not for the multi element analysis.
- Detection limit is up to parts per million.
- High resolution.
- High sensitivity.
DISADVANTAGES
- Requires large samples.
- Only powder forms are analysed by the XRF.
- Not used for the isotopic analysis.
APPLICATIONS
- Used in the soil analysis.
- Used in the trace metal analysis.
- Used the surface metal analysis.
- Used in the petroleum industry.
- Used in the environmental studies.
- Used in the qualitative analysis of the compounds.
- Used in the ores analysis.
REVIEW QUESTIONS
- What is the difference between the fluorimetry and X-ray fluorimetry?
- What is the principle involved in the X-ray fluorimetry?
- What is the theory involved in the X-ray fluorimetry?
- What are different instrumental components of the X-ray fluorimeter?
- What are the advantages and limitations of the X-ray fluorescence spectroscopy?
- What are the applications of X-ray fluorescence spectroscopy?
Comments