Free pharmacy material


Thin Layer Chromatography
INTRODUCTION
In 1949, Mein hard and Hall first proposed Thin Layer Chromatography (TLC) method by using the starch as binder to separate the inorganic ions. Kirchner proposed the conventional ascending TLC method. TLC is a solid-liquid technique in which two phases are there: one is stationary phase and other is mobile phase. The TLC principle was first introduced by the Izmailov and Shraiber. Stahl was first designed the standard equipment for the TLC. It is similar to that of the paper chromatography.
PRINCIPLE
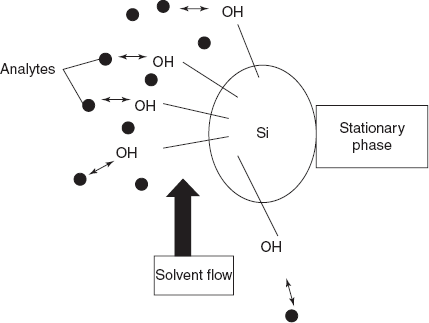
The main principle involved in the TLC is the mobile phase flows through the stationary phase where a solid or liquid is supported on the solid and carries the components of mixture which will be separated into individual analytes. The separation is based upon the migration of the analytes between the stationary phase and mobile phase. The components which have more affinity towards the stationary phase elute later and which have less affinity elutes first.
There are mainly two types of TLC methods. They are as follows:
- Normal phase TLC: Where the stationary phase is polar and the mobile phase is non-polar.
- Reverse phase TLC: Where the stationary phase is non-polar and the mobile phase is the polar.
THEORY
The theory of the TLC is as follows: the plastic or glass or aluminium sheet is coated with a layer of silica gel or alumina powder. The silica gel used is in the form of silicon dioxide.
Silicon atoms are joined through the oxygen atoms in a giant covalent structure.
A very small amount of a sample solution of the substance to be analysed is applied as small spot with a capillary tube on TLC plate 1 cm from the bottom. Then this TLC plate is developed in a chamber containing mobile phase where the different types of solvents are used. Then the mobile phase moves up through the TLC plate by capillary action which dissolves the sample and moves up based on the inter molecular forces between the stationary phase and mobile phase with sample components. The free particles are completely dissolved in the liquid or gaseous mobile phase. Then the adsorbed particles are bound to the stationary phase. This can be attained by the equilibrium between the mobile phase and stationary phase. The equilibrium between the mobile phase and the stationary phase depends on the following:
- The polarity and size of the sample.
- The polarity of the stationary phase molecules.
- The polarity of the mobile phase.
Mechanism of the solvent flow through the stationary phase
THE PROCESS OF TLC
- Preparation of the plate: The preparations of the TLC plates are done by the slurry of the adsorbent for example, silica gel, cellulose powder, etc., is spread uniformly over the plate by means of commercially available spreaders. The adsorbent layer should have the thickness of 150–250 μm. Then these plates are dried at 80–90 °C for 30 min in hot air oven or air drying over night. There are different methods for applying the slurry of adsorbent on the plate. They are as follows:
- Pouring: This is nothing but the direct pouring of the slurry on the plate and then uniformly spreader by revolving the plate.
- Pouring technique
- Spreading: In this, the slurry is applied with the help of the applicator and then moved over the plate and uniformly spread.
Spreading technique
- Dipping: This method was first proposed by the PEIFER. In this method, the plates are dipped in the slurry.
- Dipping technique
Spraying: This method was first proposed by the REITSEMA. This is carried out by the spraying with the help of sprayer.
- Precoated plates: In this method, the adsorbents are precoated on glass or plastic sheets. The main advantage is ready to use. The main disadvantage is these are very expensive.
- Activation of the plates: These plates are activated by placing the adsorbent-coated plates in the oven at 50-60 °C for 15-20 min. This step is mainly to remove the water molecules present in the slurry.
- Marking the plate: The plate is marked with the help of pencil leaving 2-4 cm from the bottom. The marking is also done by the marker pen instead of pencil to get accurate results.
- Spotting of the sample: The sample applied with the help of micro syringe or with the help of micro capillary to get the minute sample is spotted properly. The capillary is filled with the sample solution where the sample is dissolved in the appropriate solvent and then allow the solvent to evaporate from the spot completely.
- Preparing the development tank: The plates of the TLC are generally developed by the ascending technique where the plates are placed in the developing solvent that is mobile phase. The tank is saturated with the solvent vapours. The care should be taken for the complete closing of the chamber with the help of the lid. The development of the plates is done by the immersing in the tank which contains the mobile phase. The plates are immersed in such a way that the 0.5 cm of the plate is dipped in the solvent.
- Development technique
- Solvents (mobile phase): The selection of solvent system is mainly based on the polarity and nature of the sample to be analysed. Generally used solvents in the TLC are the following:
- Very polar solvents: methanol, ethanol and isopropanol.
- Moderately polar solvents: acetonitrile, ethyl acetate, chloroform and diethyl ether.
- Non-polar solvents: cyclohexane, petroleum ether, hexane and pentane
The solvent selection depends on the following factors:
- Nature of the sample.
- Nature of the stationary phase.
- Mode of chromatography.
- Mode of separation.
Drying of the plate: The plate developed in the development tank is dried by means of exposing to the air or placing it in the oven at 50-60 °C for 30 min.
Detection of components: The spots dried are detected by the exposure to the UV light or by treating the spots with the chemical reagents. First the spots formed are lined with the help of the pencil. The boundary of the spot is marked with pencil. Then the spot is detected with the different methods. They are the following:
- Iodination: The plates are placed in the chamber containing the iodine crystals.
- Ninhydrin technique: Where the spots are sprayed with the ninhydrin reagent. This method is mainly used for the determination of the compounds containing amine as a functional group.
- By using the fluorescent radiation.
- By using the UV radiation.
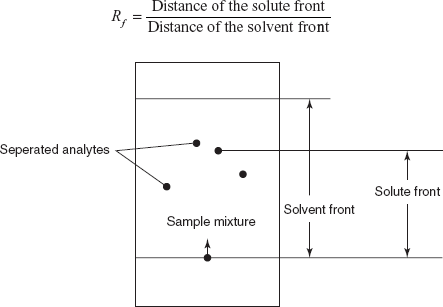
- Rf measurement: The Rf is defined as the ratio of the distance of the centre of the spot moved to that of the solvent front moved.
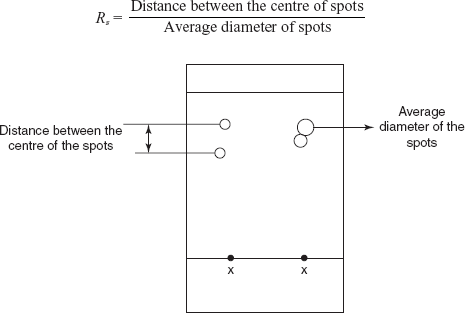
Resolution of the spots
Resolution is defined as the separation of the two analytes on TLC chromatogram which is known as resolution commonly denoted as Rs.
Accuracy of the spots
Flow constant is determined by taking the migration rate of the solvent front which is denoted by the K
K = Zf2/t
where K is the flow constant; Z is the distance between the solute front and solvent front; t is the development time.
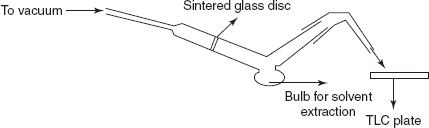
Recovery of the components: The recovery of the sample is done by using the Craig tube for the extraction of the solvent from the powder and to remove the adsorbent. Then the other method used is the removing of the zones by means of the spatula and followed by the extraction with solvent.
Recovery of the samples
ADVANTAGES OF TLC OVER OTHER TECHNIQUES
- Simple equipment compared to other methods.
- The sample preparation is simple.
- Mobile phase required is less.
- Less time consuming.
- There is wide choice of selection when compared to the other methods.
- Detected easily by using different methods.
- Low cost.
- Reliability is high.
- High sensitivity.
- High resolution.
- Inert stationary phase.
DISADVANTAGES OF TLC
- Over large spots formation.
- Uneven migration of the solvent front.
- Some spots are appeared as the streaked spots.
TROUBLE SHOOTING IN TLC
- Over migration of the spots: decrease the polarity of the solvent.
- Tailing of the peaks: reduce the concentration of the sample or decrease the acidity or basicity.
- Distorted solvent front: equilibrate the mobile phase.
APPLICATIONS
- Used in the determination of the impurities from which the purity of the compounds are determined.
- Used in the identification of the compounds:
- - Stramonium leaf constituents are identified.
- - Fixed oils are identified.
- - Steroids are identified.
- - Phenothiazines are identified.
- Used in the determination of the analgesics.
- Example: Paracetamol, analgin.
- Used in the determination of the biologically active substances.
- Used in the determination of preservatives, oxidants, surfactants and fixed oils.
- Used in the determination of pesticides in the water.
- Used in the determination of inorganic ions.
- Used in the determination of the reaction rate.
- Used in the determination of amino acids and peptides.
- Used in the inks analysis
REVIEW QUESTIONS
- What are the solvents used in the development of the TLC?
- List out the advantages of the TLC?
- Write about the recovery of the samples in TLC.
- How do you develop the plates in TLC?
- How do you determine the flow constant in the TLC?
- What is the different detection methods used for detection of spots in the TLC?
- What are the different methods used for the TLC plates preparation?
Comments