Free pharmacy material















Sulphonamides, Sulphones, and Dihydrofolate Reductase Inhibitors
The sulphonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. The sulphonamides and sulphone antibacterials as well as the 2,4-diaminopyrimidine antifolates continue to be successful chemotherapeutic agents.
24.1 SULPHONAMIDES
The sulphonamide drugs were the first effective chemotherapeutic agents to be employed systemically for the prevention of bacterial infections in human beings. These are totally synthetic substances that are produced by relatively simple chemical synthesis. Sulphonamide drugs are a group of synthetic antimicrobial drugs that have a broad spectrum of use with respect to grampositive as well as gram-negative micro-organisms. They were introduced into medical practice even before the discovery of penicillins. Sulphonamide drugs are derivatives of sulphanilamide. The advent of penicillin and subsequently of other antibiotics has diminished the usefulness of sulphonamides.
Classification
- Absorbed and excreted rapidly: Sulphisoxazole, Sulphamethoxazole, and Sulphadiazine
- Poorly absorbed (active in bowel lumen): Sulphasalazine
- Topically used: Sulphacetamide, Silver sulphadiazine, Mefenide
- Long-acting: Sulphadoxine
Mechanism of action:
Sulphonamides are structurally similar to PABA. They compete with PABA for the enzyme dihydrofolate synthase and block the synthesis of DHFA (dihydrofolic acid), in turn tetrahydrofolic acid (THFA) and folate cofactor. Folate cofactor acts as 1-carbon donor for the synthesis of nucleic acid (DNA). The result of blocking the biosynthesis of folate coenzymes in bacteria, for example, is that growth and cell division are stopped. Sulphonamides are, thus, bacteriostatic drugs. Since mammalian cells use preformed folates from the diet, and since most bacteria cannot use preformed folates and must synthesise their own folic acid, the sulphonamides, therefore, demonstrate a selective toxicity to bacteria.
Uses
- Because sulpha drugs concentrate in the urine before being excreted, treating urinary tract infections (UTI) is one of their most common uses. The sulphonamide antibacterials are primarily used for the treatment of uncomplicated urinary tract infections caused by E. coli,Klebsiella, Enterobacter, and Proteus, and only seldom for middle ear infections caused byHaemophilus influenzae. Sulphisoxazole and sulphamethoxazole are the major therapeutic drugs for UTI.
- The sulphonamides are also used for the prophylaxis of recurrent rheumatic fever associated with streptococcal infection as well as for the prophylaxis of Neisseria meningitis.
- Sulphonamides can be co-administered with antifolate antibacterials, usually with trimethoprim for antibacterial activity and with pyrimethamine for antimalarial activity.
- Topically, sulphonamides (silver sulphadiazine) are useful in burn therapy.
- Sulphasalazine is useful for the treatment of ulcerative colitis.
Side-effects
Sulphanilamide causes severe kidney damage from crystals of sulphanilamide forming in the kidney. Sulphanilamide and their metabolites are excreted entirely in the urine. Unfortunately, sulphanilamide is not very water-soluble and this leads to crystalluria. Crystalluria can be overcome by:
- Greatly increasing urine flow
- Raising the pH of the urine to 10.4 with sodium bicarbonate
- Lowering pKa, which is achieved by attaching electron-withdrawing heterocycle at N1 position of sulphanilamide
Gerhard Domagk and Jacques and Therese Trefouel (1935) are generally credited with the discovery of sulphanilamide as a chemotherapeutic agent. Domagk was awarded the Nobel Prize for his work.
SAR of sulphonamide
- The amino and sulphonyl groups on the benzene ring are essential and should be in 1,4-position.
- The N4 amino group could be modified to produce prodrugs, which are converted to free amino function in vivo.
- Replacement of benzene ring by other ring systems or the introduction of additional substituents on it decreases or abolishes activity.
- Exchange of the -SO2NH group by -CONH reduces the activity.
- Substitution of heterocyclic aromatic nuclei at N1 yields highly potent compounds.
- N1-Disubstitution in general leads to inactive compounds.
Synthesis
General methods of preparation
Acetanilide on treatment with chlorosulphonic acid gives sulphonyl chloride derivative, which on reaction with appropriate primary amine gives sulphonamide derivative. Saponification gives the final target compound.
Sulphisoxazole N1-(3,4-Dimethyl-5-isoxazolyl) sulphanilamide
Sulphisoxazole is synthesised by reacting 4-acetylaminobenzenesulphonyl chloride with 5-amino-3,4-dimethylisoxazol, which is in turn synthesised by heterocyclization of 2-methylacetylacetonitrile (prepared by Claisen condensation of propionitrile and ethylacetate in presence of base) with hydroxylamine, and subsequent acidic hydrolysis (hydrochloric acid) of the protective acetyl group in the resulting product.
Sulphamethoxazole N1-(5-Methyl-3-isoxazolyl) sulphanilamide
Sulphamethoxazole is synthesised by a completely analogous scheme, except by using 3-amino-5-methylisoxazol as the heterocyclic component.
It is used only in combination with trimethoprim (co-trimoxazole).
Sulphamoxole 4-Amino-N-(4,5-dimethyl-1,3-oxazol-2-yl)benzenesulphonamide
Sulphadiazine N1-2-Pyrimidinyl sulphanilamide
Sulphadiazine is synthesised by a completely analogous scheme, except by using 2-amino-pyrimidine as the heterocyclic component.
Sulphadimidin 4-Amino-N-(4,6-dimethylpyrimidin-2-yl)benzenesulphonamide
Sulphasalazine 2-Hydroxy-5-[[4-[(2-pyridinyl amino) sulphonyl] phenyl] azo] benzoic acid
Sulphapyridine on treatment with sodium nitrite and hydrochloric acid gives diazonium salt intermediate. This, on coupling with salicylic acid, affords sulphasalazine.
Sulphasalazine is poorly absorbed from the small intestine, so that the drug passes into the colon where the bacterial enzymes release both 5-amino salicylic acid and sulphapyridine from the drug. It has a suppressive effect on ulcerative colitis. Sulphapyridine decreases anaerobic bacteria and 5-amino salicylate inhibits prostaglandin synthesis.
Sulphacetamide N1-Acetylsulphanilamide
Sulphacetamide is synthesised by reacting 4-aminobenzenesulphonamide with acetic anhydride and subsequent selective, reductive deacylation of the resulting acetamide using a system of zinc-sodium hydroxide.
It is mainly used locally for ophthalmologic infections.
Sulphaguanidin 4-Amino-N-[amino(imino)methyl]benzenesulphonamide
Silver sulphadiazine 4-Amino-N-(2-pyrimidinyl)benzenesulphonamide silver salt
It combines in one compound the antibacterial properties of silver ion and sulphadiazine, and is especially effective in the treatment of burn infections. Silver sulphadiazine is typically delivered in a one-per-cent solution suspended in a water-soluble base.
Mefenide α-Amino-p-toluene sulphonamide
Mefenide is synthesised by reduction of 4-cyanobenzenesulphonamide.
It is applied locally for burn infections. Although it is a sulphonamide, the para substituent differs from the sulpha drug and its mechanism of action is much different.
Sulphadoxine N1- (5,6- Dimethoxy-4-pyrimidinyl sulphanilamide)
Sulphadoxine is synthesised by the standard scheme from 4-acetylaminobenzenesulphonyl chloride and 4-amino-5,6-dimethoxypyrimidine. However, 4-amino-5,6-dimethoxypyrimidine is synthesised from methyl ester of methoxyacetic acid. Interacting this with dimethyloxalate in the presence of sodium methoxide gives the methoxy derivative, and the pyrolysis of this compound gives the dimethyl ester of methoxymalonic acid. Reacting this with ammonia gives the diamide of methoxymalonic acid. Heterocyclization of the resulting product by a reaction with formamide in the presence of sodium ethoxide gives 4,6-dioxy-5-methoxypyrimidine, which is then transformed to 4,6-dichloro-5-methoxypyrimidine. The resulting 4,6-dichloro-5-methoxypyrimidine undergoes a reaction with ammonia to make 4-amino-6-chloro-5-methoxypyrimidine, and the resulting compound is then reacted with sodium methoxide to make the desired 5,6-dimethoxy-5-aminopyrimidine. Reacting this with 4-acetylaminobenzenesulphonyl chloride and subsequent hydrolysis of the acetyl group gives sulphadoxine.
Its principal use is in the prophylaxis or suppression of malaria caused by chloroquine-resistant P.falciparum. It is used only in combination with pyrimethamine.
24.2 SULPHONES
It has been estimated that there are about 11 million cases of leprosy in the world, of which about 60 per cent are in Asia (with 3.5 million in India alone). Lesions of the skin, loss of sensitivity to pain, and superficial nerves are the three main signs of leprosy, a disease caused by the mycobacteriaMycobacterium leprae. This disease is extremely infectious.
Diamino diphenyl sulphones (dapsone) is used for the treatment of infection caused byMycobacterium leprae. Chemotherapy of leprosy consisted of taking dapsone, which gave good clinical results. However, because of the primary and secondary resistance that originated from prolonged use, it is now necessary to use a certain combination of drugs. Currently, dapsone is used along with rifampicin and clofazimine. Ethionamide is also prescribed.
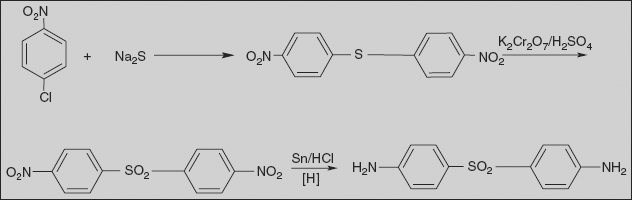
Dapsone 4, 4’-Sulphonyl bis benzenamine
Reacting 4-chloronitrobenzene with sodium sulphide gives 4,4’-dinitrodiphenylthioester, and oxidation of the sulphur atom in this compound using potassium dichromate in sulphuric acid gives 4,4’-dinitrodiphenylsulphone. Reduction of the nitro group in the resulting compound using tin dichloride in hydrochloric acid makes the desired dapsone.
It has mechanism of action similar to that of sulphonamides. It is used in the treatment of both lepromatous and tuberculoid types of leprosy. Dapsone is used in combination with rifampicin and clofazimine. Dapsone is also the drug of choice for dermatitis herpetiformis, with pyrimethamine for the treatment of malaria, with trimethoprim for Pneumocystis carinii pneumonia (PCP), and has been used for rheumatoid arthritis.
It is used prophylactically to prevent Pneumocystis pneumonia and toxoplasmosis in patients unable to tolerate trimethoprim with sulphamethoxazole. Dapsone is also used to treat Brown recluse spider bites. In December 2008, a 5 per cent dapsone gel called Aczone was intoduced to the prescription market as a treatment for moderate to severe acne.
24.3 DIHYDROFOLATE REDUCTASE (DHFR) INHIBITORS
2,4-Diamino pyrimidine derivatives like trimethoprim and pyrimethamine inhibit DHFR of bacteria and Plasmodium, respectively. The enzyme DHFR converts dihydrofolic acid to tetrahydrofolic acid, in turn to folate cofactors. These drugs block the DNA synthesis and cell division.
Sulphonamides and trimethoprim are used to treat and prevent infections. The sulphadiazine-and-trimethoprim combination is used to treat urinary tract infections. The sulphamethoxazole-and-trimethoprim (co-trimoxazole) combination is used to treat infections such as bronchitis, middle ear infection, urinary tract infection, and traveller’s diarrhoea. It is also used for the prevention and treatment of Pneumocystis carinii pneumonia (PCP).
Trimethoprim 2,4-Diamino-5-(3’,4’,5’-trimethoxy benzyl)pyrimidine
Condensation of 3,4,5-trimethoxybenzaldehyde with 3-ethoxypropionitrile gives the corresponding benzylidene derivative, which upon direct reaction with guanidine gives trimethoprim.
It is used along with sulphamethoxazole for bacterial infections.
Pyrimethamine 2,4- Diamino-5-(p-chlorophenyl)-6-ethylpyrimidine
Pyrimethamine is synthesised from 4-chlorobenzycyanide, which upon condensation with ethyl ester of propionic acid in the presence of sodium methoxide gives the β-ketonitrile. Reacting this with diazomethane/ethylorthoformate gives a methoxymethylene derivative, which upon heterocyclization in pyrimidine using guanidine as the binucleophile forms the desired pyrimethamine.
It is used in combination with sulphadoxine for the treatment of malaria.
24.4 NEWER DRUG
Brodimoprim: 5-[(4-Bromo-3,5-dimethoxyphenyl)methyl] pyrimidine-2,4-diamine
It is a structural derivative of trimethoprim. In brodimoprim, the 4-methoxy group of trimethoprim is replaced with a bromine atom. As trimethoprim, brodimoprim is a selective inhibitor of bacterial dihydrofolate reductase.
FURTHER READINGS
1. Annual Reports in Medicinal Chemistry, September 30, 2003, Elsevier.
2. King, F.D., G. Lawton, and A.W. Oxford, May 2004. Progress in Medicinal Chemistry, Elsevier.
3. Martin, Yvonne Connolly, Eberhard Kutter, Volkhard Austel, and Marcel Dekker, 1989. Modern Drug Research.
MULTIPLE-CHOICE QUESTIONS
- Sulphonamides block the synthesis of
- PABA
- DFA
- DHFA
- TFA
- Topically used sulphonamide is
- Sulphadoxine
- Sulphamethoxazole
- Silver sulphadiazine
- Dapsone
- Which of the following statements refers to dapsone?
- Not much used due to toxicity
- Inhibits folate synthesis in bacteria
- Is reduced by intestinal bacteria to a salicylate
- Is reduced by intestinal bacteria to a sulphonamide
- Co-trimoxazole is a combination of
- Sulphadiazine and trimethoprim
- Sulphamethoxazole and trimethoprim
- Sulphamethoxazole and sulphadoxine
- Sulphamethoxazole and pyrimethamine
- Dapsone is used primarily for the treatment of
- Tuberculosis
- Leprosy
- Malaria
- Urinary tract infection
- One of the following does not contain pyrimidine in its structure:
- Sulphadiazine
- Sulphadoxine
- Pyrimethamine
- Dapsone
- The long-acting sulphonamide is
- Sulphamethoxazole
- Sulphadiazine
- Sulphadoxine
- Sulphacetamide
- Treatment of malaria is achieved with the combination of pyrimethamine with all of the following except:
- Sulphadoxine
- Dapsone
- Trimethoprim
- Sulphadiazine
- Which of the following statements is true?
- Sulphanilamide is soluble in water.
- Sulphonamides are retained in urine before excretion.
- The terminal amino function is not required for activity.
- Sulphonamides are useful for antitubercular therapy.
- Traveller’s diarrhoea is treated with
- Sulphadiazine
- Co-trimoxazole
- Dapsone
- Pyrimethamine
QUESTIONS
- What is crystalluria? How can it be prevented?
- Discuss the mechanism of action of sulpha drugs.
- Write short notes on combination of drugs and their uses.
- Identify the heterocyclic ring system present in sulphadiazine, sulphasalazine, and sulphadoxine.
- Explain how sulphasalazine exhibits its action.
- Discuss briefly the SAR of sulphonamides.
- Write the synthetic protocol for dapsone, pyrimethamine, sulphadiazine, and sulphametho